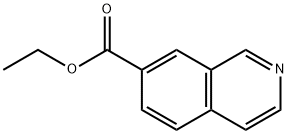
Ethyl 7-isoquinolinecarboxylate synthesis
- Product Name:Ethyl 7-isoquinolinecarboxylate
- CAS Number:407623-83-0
- Molecular formula:C12H11NO2
- Molecular Weight:201.22
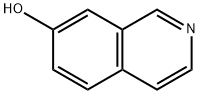
7651-83-4
154 suppliers
$24.00/100mg
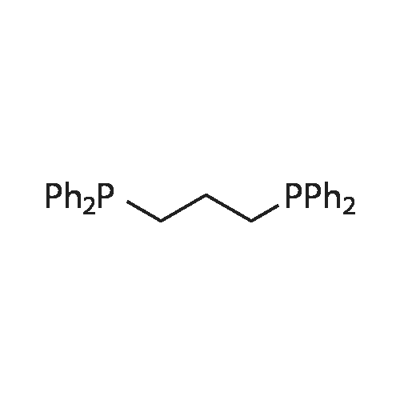
6737-42-4
418 suppliers
$6.00/5g

407623-83-0
46 suppliers
$67.00/100mg
Yield:-
Reaction Conditions:
with pyridine;trifluorormethanesulfonic acid;triethylamine;carbon monoxide;tetrakis(triphenylphosphine)palladium (0) in ethanol;dichloromethane;N,N-dimethyl-formamide;
Steps:
205.1 Step 1
Step 1 Synthesis of 7-ethoxycarbonylisoquinoline: 0.89 g (6.1 mmol) of 7-hydroxyisoquinoline was dissolved in 15 ml of dichloromethane. 1.1 ml of pyridine and 1.3 ml (7.7 mmol) of anhydrous trifluoromethanesulfonic acid were added to the obtained solution under cooling with ice, and they were stirred at 0° C. for 1 hour. After the treatment with dichloromethane as the extracting solvent by an ordinary method, the obtained crude product was purified by the silica gel column chromatography to obtain an oily product. This oily product was dissolved in 10 ml of DMF. 0.4 ml (2.9 mmol) of triethylamine, 50 mg (0.04 mmol) of tetrakistriphenylphosphine palladium, 16 mg (0.04 mmol) of 1,3-bis(diphenylphosphino)propane and 5 ml of ethanol were added to the obtained solution, and they were stirred in the presence of carbon monoxide at 70° C. overnight. The solvent was evaporated, and the residue was purified by the silica gel column chromatography to obtain the title compound. Yield: 196 mg (0.97 mmol) (16%) MS (ESI, m/z) 202 (MH+) H-NMR (CD3Cl) δ 1.48 (3H, t), 4.47 (2H, q), 7.70 (1H, d), 7.88 (1H, d), 8.30 (1H, dd), 8.62 (1H, d), 8.74 (1H, s), 9.37 (1H, s)
References:
US2003/186969,2003,A1