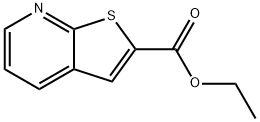
ETHYL THIENO[2,3-B]PYRIDINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL THIENO[2,3-B]PYRIDINE-2-CARBOXYLATE
- CAS Number:59944-78-4
- Molecular formula:C10H9NO2S
- Molecular Weight:207.25
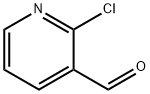
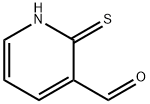
36404-88-3
330 suppliers
$5.00/1g
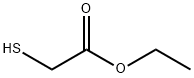
623-51-8
247 suppliers
$7.00/25g
![ETHYL THIENO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/59944-78-4.gif)
59944-78-4
43 suppliers
$85.00/500mg
Yield:59944-78-4 59%
Reaction Conditions:
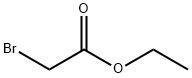
Stage #1: ethyl 2-sulfanylacetatewith sodium ethanolate in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2: 2-chloro-3-carbaldehydepyridine in N,N-dimethyl-formamide at 120;
Steps:
1-A; I Example 1-A
[0544] To a solution of ethyl thioglycolate (11.14 g, 92.8 mmol) in 400 mL of DMF was added NaOEt (14.5 g, 185.7 mmol) by portion wise. The resulting mixture was stirred for 30 min at 0° C. And then I-1 (10 g, 71.4 mmol) was added to the solution by portion wise. The mixture was stirred at 120° C. overnight. The reaction mixture was cooled to rt., diluted with water (300 mL), extracted with EtOAc (300 mL×3), the combined organic layers were washed with brine, dried over anhydrous Na2SO4 and concentrated. The residue was washed with petroleum ether to afford I-2 (8.7 g, 59% yield) as a pale brown solid. 1H NMR (CDCl3, 400 MHz) δ 8.68 (dd, J=1.6, 4.4 Hz, 1H), 8.16 (dd, J=1.6, 8.0 Hz, 1H), 8.00 (s, 1H), 7.36 (m, 1H), 4.43 (q, J=7.2 Hz, 2H), 1.42 (t, J=7.2 Hz, 3H). MS (ESI) m/z [M+H]+ 208.0.
References:
US2014/94456,2014,A1 Location in patent:Paragraph 0543-0544
![ETHYL 3-AMINOTHIENO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/52505-46-1.gif)
52505-46-1
97 suppliers
$20.00/1g
![ETHYL THIENO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/59944-78-4.gif)
59944-78-4
43 suppliers
$85.00/500mg