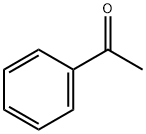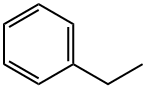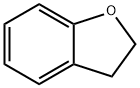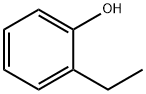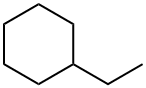
Ethylcyclohexane synthesis
- Product Name:Ethylcyclohexane
- CAS Number:1678-91-7
- Molecular formula:C8H16
- Molecular Weight:112.21
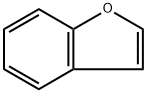
271-89-6
266 suppliers
$9.00/1g

1678-91-7
205 suppliers
$36.00/25mL
Yield:1678-91-7 83.2%
Reaction Conditions:
with rhodium contaminated with carbon;N-methyldiethanolamine trifluoromethanesulfonate;hydrogen at 150; under 37503.8 Torr; for 6 h;Autoclave;
Steps:
5 Example 5
1 mmol of benzofuran, 0.1 g of commercial Rh/C (water content 55-60%) catalyst, 2 g of ionic liquid ([BHEM] [OTf]), 0.1770 g of n-dodecane and magnetons were added to contain polytetrafluoroethylene Tighten the seal in a lined stainless steel autoclave. The autoclave was then purged three times with high purity hydrogen to blow out the air in the autoclave, and after the last gas exchange, 5 MPa of hydrogen was injected into the autoclave. The reaction kettle was placed in a high-pressure reaction furnace, and the temperature was raised to a set temperature of 150 ° C, magnetic stirring was turned on, and the stirring speed of the magnet was set at 570 rpm, and the timing was started. After reacting for 6 hours, the reaction vessel was placed in ice water for cooling. After being completely cooled, the high-pressure hydrogen remaining in the reaction vessel was slowly discharged, and after the hydrogen in the autoclave was purged with nitrogen, the reaction vessel was opened, and the reaction product was extracted with 8 ml of methyl t-butyl ether. The conversion of benzofuran was 100%, and the yield of ethylcyclohexane was 83.2%.
References:
Chinese Academy Of Sciences Process Engineering Institute;Zhang Suojiang;Zhou Qing;Cai Guangming;Yang Shaoqi;Li Xiaoqian;Lv Xingmei CN109704902, 2019, A Location in patent:Paragraph 0035; 0036
931-48-6
73 suppliers
$50.00/1g

1678-91-7
205 suppliers
$36.00/25mL
721-04-0
64 suppliers
$60.00/100mg
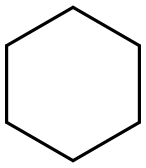
110-82-7
769 suppliers
$10.00/25g

1678-91-7
205 suppliers
$36.00/25mL
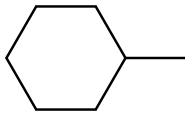
82166-21-0
0 suppliers
inquiry