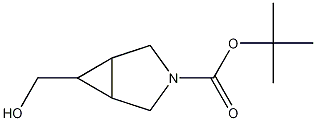
exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol synthesis
- Product Name:exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol
- CAS Number:419572-18-2
- Molecular formula:C11H19NO3
- Molecular Weight:213.27

24424-99-5
![(1R,5S,6R)-3-azabicyclo[3.1.0]hexan-6-ylmethanol](/CAS/GIF/134575-13-6.gif)
134575-13-6
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
The general procedure for the synthesis of tert-butyl rel-(1R,5S,6r)-6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate from di-tert-butyl dicarbonate and exo-3-azabicyclo[3.1.0]hexan-6-ylmethanol was as follows: (1R,5S,6R)-3-azabicyclo[3.1.0]hexan-6-ylmethanol (10 g. 88.4 mmol) was dissolved in a solvent mixture of dioxane and water (3:2, v/v), followed by addition of sodium hydroxide (4.2 g, 106 mmol) and di-tert-butyl dicarbonate (28.9 g, 132.6 mmol). After the reaction was carried out for 10 h, 50 mL of water was added to the reaction mixture. The reaction mixture was extracted with ethyl acetate (150 mL each time, 3 times). The organic phases were combined, washed with saturated sodium chloride solution (50 mL each time, 2 times), then dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and the resulting residue was purified by silica gel column chromatography using elution system C. The target product (1R,5S,6R)-tert-butyl 6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate (16.9 g, 89.9% yield) was obtained as a pale yellow liquid.

24424-99-5
871 suppliers
$13.50/25G
![(1R,5S,6R)-3-azabicyclo[3.1.0]hexan-6-ylmethanol](/CAS/GIF/134575-13-6.gif)
134575-13-6
85 suppliers
$60.00/100mg
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg
Yield: 89.9%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20 - 30; for 10 h;
Steps:
31.1 Example 31 Step 1 (1R,5S,6R)-tert-Butyl 6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate
(1R,5S,6R)-3-Azabicyclo[3.1.0]hexan-6-ylmethanol 31a (10 g, 88.4 mmol) was dissolved in a mixed solvent of dioxane and H2O (V/V=3/2), followed by addition of sodium hydroxide (4.2 g, 106 mmol) and di-tert-butyl dicarbonate (28.9 g, 132.6 mmol).
After reacting for 10 hours, 50 mL of H2O was added to the reaction mixture.
The reaction mixture was extracted with ethyl acetate (150 mLx3).
The organic phases were combined, washed with saturated sodium chloride solution (50 mLx2), dried over anhydrous sodium sulfate, and filtered.
The filtrate was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography with elution system C to obtain the title product (1R,5S,6R)-tert-butyl 6-(hydroxymethyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate 31b (16.9 g, yield 89.9%) as a light yellow liquid.
References:
Location in patent:Paragraph 0306; 0307
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
13 suppliers
inquiry
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
124 suppliers
$29.00/100mg
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg
![(1α,5α,6α)-3-Azabicyclo[3.1.0]hexan-6-ylmethanol hydrochloride](/CAS/20211123/GIF/185561-91-5.gif)
185561-91-5
10 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg
![3-Oxabicyclo[3.1.0]hexane-6-carboxylicacid,2,4-dioxo-,(1alpha,5alpha,6alpha)-(9CI)](/CAS/GIF/187681-87-4.gif)
187681-87-4
13 suppliers
inquiry
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg