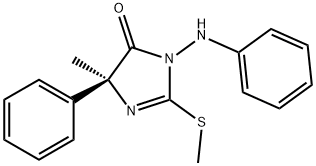
FENAMIDONE synthesis
- Product Name:FENAMIDONE
- CAS Number:161326-34-7
- Molecular formula:C17H17N3OS
- Molecular Weight:311.4
![(+)-[methyl (2S)-2-isothiocyanato-2-phenylpropionate]](/CAS/20200119/GIF/252268-16-9.gif)
252268-16-9
0 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g

161326-34-7
62 suppliers
$55.60/100mg-r
Yield:161326-34-7 58%
Reaction Conditions:
with potassium tert-butylate;phenylhydrazine in tetrahydrofuran;water;toluene
Steps:
19 Preparation of (+)-(4S)-4-methyl-2-methylthio-4-phenyl-1-phenylamino-2-imidazolin-5-one (Compound 501):
EXAMPLE 19 Preparation of (+)-(4S)-4-methyl-2-methylthio-4-phenyl-1-phenylamino-2-imidazolin-5-one (Compound 501): 682 g (3.08 mol) of methyl (+)-(2S)-2-phenyl-2-(isothiocyanato)propionate, dissolved in 4 liters of anhydrous tetrahydrofuran, are introduced into a 20 liter reactor through which passes a stream of argon. Cooling is carried out to 15° C. 343 g (3.08 mol) of phenylhydrazine, dissolved in 2 liters of tetrahydrofuran, are run in over 30 minutes, the temperature being maintained between 15° C. and 18° C. The mixture is kept stirring for 40 minutes and then cooled to 0° C. A solution of 346 g (3.08 mol) of potassium tert-butoxide in 4 liters of tetrahydrofuran is run in over 1 hour, the temperature being maintained at 0° C. The mixture is stirred for a further 2 hours at 0° C. and the formation of a pale-pink precipitate is observed. 218 ml (3.39 mol) of methyl iodide are run in over 15 minutes, the temperature being maintained between 0° C. and 3° C., and the temperature is then allowed to rise to room temperature while continuing to stir for 2 hours. The reaction mixture is poured onto 5 liters of water. After separating, the aqueous phase is extracted with 3 times 3 liters of ethyl acetate. The combined organic phases are washed with 5 liters of water, dried over magnesium sulfate and the concentrated under reduced pressure. 1099 g of a brown solid are obtained. The latter is recrystallized from 2 liters of toluene. There are obtained, after drying, 555 g of (+)-(4S)-4-methyl-2-methylthio-4-phenyl-1-phenylamino-2-imidazolin-5-one in the form of an off-white solid melting at 138° C.; yield=58%; [α]D27° C. =+61.1° (+ or -2.9°) (c=0.86 in ethanol); degree of enantiomeric excess (e.e)>98%.
References:
Rhone-Poulenc Agrochimie US6002016, 1999, A
![(+)-[methyl (2S)-2-isothiocyanato-2-phenylpropionate]](/CAS/20200119/GIF/252268-16-9.gif)
252268-16-9
0 suppliers
inquiry
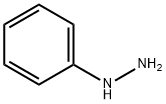
100-63-0
382 suppliers
$10.00/1g

74-88-4
356 suppliers
$15.00/10g

161326-34-7
62 suppliers
$55.60/100mg-r