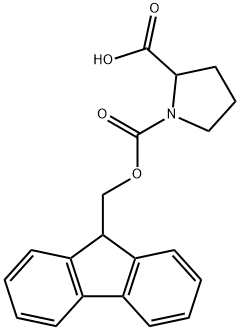
FMoc-DL-proline synthesis
- Product Name:FMoc-DL-proline
- CAS Number:144829-96-9
- Molecular formula:C20H19NO4
- Molecular Weight:337.37
Yield:144829-96-9 620 mg
Reaction Conditions:
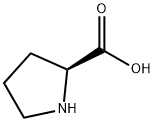
Stage #1: rac-Pro-OHwith sodium carbonate in water at 20; for 0.0833333 h;
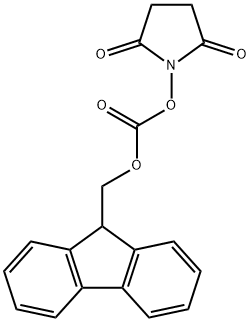
Stage #2: N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide in tetrahydrofuran at 20; for 2 h;
Steps:
0246-1 0246-1
0246-1
Water (5 mL) was added to pyrrolidine-2-carboxylic acid (230 mg) and sodium carbonate (500 mg), followed by stirring at room temperature for 5 minutes.
A tetrahydrofuran solution (5 mL) of (9-fluorenylmethyl)succinimidyl carbonate (720 mg) was added to the reaction mixture, followed by stirring at room temperature for 2 hours.
Ethyl acetate was added to the reaction mixture.
The aqueous layer was neutralized by the addition of hydrochloric acid, extracted three times with ethyl acetate, and the organic layer was washed with a saturated sodium chloride aqueous solution.
The organic layer was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, thereby obtaining 1-(((9H-fluoren-9-yl)methoxy)carbonyl)pyrrolidine-2-carboxylic acid (620 mg).
1H-NMR (DMSO-d6) δ: 12.62 (1H, s), 7.93-7.87 (2H, m), 7.70-7.62 (2H, m), 7.45-7.29 (4H, m), 4.35-4.27 (1H, m), 4.21-4.13 (2H, m), 3.47-3.36 (2H, m), 2.59 (1H, s), 2.40-2.11 (1H, m), 1.92-1.80 (3H, m).
References:
US2015/322063,2015,A1 Location in patent:Paragraph 1380; 1381; 1382