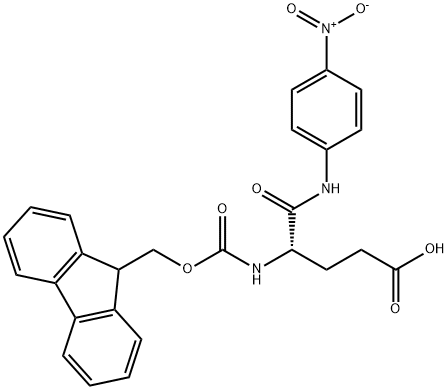
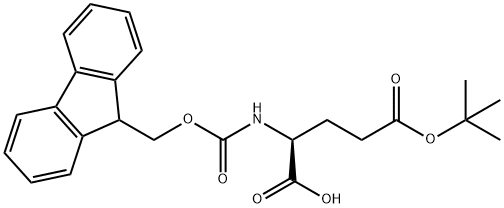
FMOC-GLU-PNA synthesis
- Product Name:FMOC-GLU-PNA
- CAS Number:185547-51-7
- Molecular formula:C26H23N3O7
- Molecular Weight:489.48
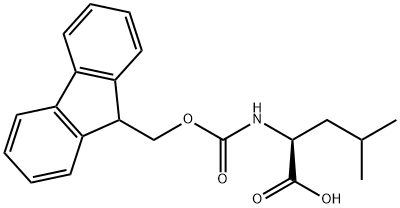
35661-60-0
522 suppliers
$5.00/1g
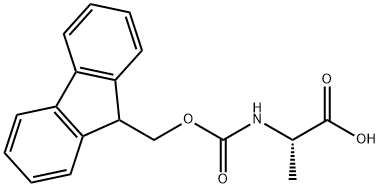
35661-39-3
501 suppliers
$5.00/1g

185547-51-7
24 suppliers
inquiry
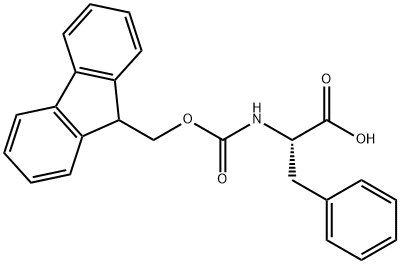
35661-40-6
386 suppliers
$5.00/5g
71989-18-9
411 suppliers
$7.00/5g
198551-00-7
27 suppliers
inquiry
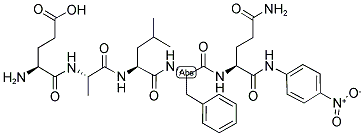
Yield:198551-00-7 63.2 mg
Reaction Conditions:
Stage #1: Fmoc-Glu-pNAwith C17H19N2O4Pol;benzotriazol-1-ol;diisopropyl-carbodiimide in N,N-dimethyl-formamide; for 0.5 h;solid phase reaction;
Stage #2: with piperidine in N,N-dimethyl-formamide; for 0.166667 h;solid phase reaction;
Stage #3: Fmoc-Leu-OH;N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine;N-Fmoc L-Phe;Fmoc-Glu(OtBu)-OHFurther stages;
Steps:
p-nitroanilide (pNA) peptide synthesis (EALFQ-pNA)
In house prepared aminomethyl polystyrene resin (162 mg, 0.1 mmol) was swollen in DMF (3 mL, 30 min) and the solvent was removed under vacuum. Fmoc-RINK amide linker (162 mg, 0.3 mmol) and HOBt (46 mg, 0.3 mmol) were dissolved in DMF (3 mL) and the mixture added to the aminomethyl polystyrene (PS) resin with subsequent addition of DIC (46 μl, 0.3 mmol). The mixture was shaken for 1 h and the solvent was removed under vacuum. The peptidyl-resin was washed thoroughly with DMF (3x10 mL) followed by DCM (3x10 mL). The Fmoc protecting group was removed according to general procedure i) with a reaction time of 10 min. Fmoc-Aa coupling was performed according to the general procedure with a reaction time of 30 min ii). The peptide was cleaved from the resin with simultaneous protecting group removal by adding a mixture of TFA, H2O and 3,6-dioxa-1,8-octanedithiol (94:3:3, v:v:v, 10 mL) to the peptidyl-resin and shaking the mixture for 2 h. The filtrate was collected and the peptide was precipitated using diethyl ether (25 mL). The solid peptide was collected by filtration and dried to afford EALFQ-pNA (63.2 mg) as an amorphous colourless solid (ca. 92 % purity as judged by the peak area in the RP-HPLC recorded at 210 nm); Rt=14.46 min; m/z (ESI-MS) 727.34 ([m+H]+) 727.34 required).
References:
Schuenemann, Katrin;Connelly, Stephen;Kowalczyk, Renata;Sperry, Jonathan;Wilson, Ian A.;Fraser, John D.;Brimble, Margaret A. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 15,p. 5018 - 5024] Location in patent:supporting information; experimental part