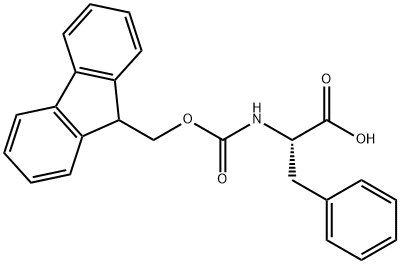
Fmoc-Phe-OH synthesis
- Product Name:Fmoc-Phe-OH
- CAS Number:35661-40-6
- Molecular formula:C24H21NO4
- Molecular Weight:387.43
63-91-2
934 suppliers
$10.00/100G
28920-43-6
553 suppliers
$5.00/5g

35661-40-6
425 suppliers
$5.00/5g
Yield:35661-40-6 82%
Reaction Conditions:
Stage #1:L-phenylalanine;(fluorenylmethoxy)carbonyl chloride with sodium carbonate in 1,4-dioxane;water at 0 - 20; for 22 h;
Stage #2: with hydrogenchloride in water
Steps:
4.2.3. 9-Fluorenylmethoxycarbonyl-l-phenylalanine (12)
To a solution of l-phenylalanine 11 (0.96 g, 5.81 mmol) in dioxane (7.5 ml) and 10% Na2CO3 aq (15 ml) was added Fmoc-Cl (1.50 g, 5.79 mmol) in dioxane (15 ml). The reaction mixture was stirred at 0 °C for 4 h and room temperature for 18 h, added water (400 ml) and washed with Et2O (2×200 ml). The aqueous layer was acidified with cHCl, filtered and dried to yield Fmoc-l-Phe 12 (1.91 g, 4.93 mmol, 82%) as a white powder; Mp 182-183 °C; 1H NMR (400 MHz, CDCl3) δH 7.78-7.14 (13H, m), 5.18 (1H, d, J=8.2 Hz), 4.70 (1H, m), 4.45 (1H, m), 4.37 (1H, m), 4.21 (1H, m), 3.20 (1H, m), 3.14 (1H, m).
References:
Ishiyama, Haruaki;Yoshizawa, Kazuaki;Kobayashi, Jun'ichi [Tetrahedron,2012,vol. 68,# 31,p. 6186 - 6192] Location in patent:experimental part
![L-Phenylalanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-, 2-propen-1-yl ester](/CAS/20210305/GIF/146549-19-1.gif)
146549-19-1
0 suppliers
inquiry

35661-40-6
425 suppliers
$5.00/5g
![methyl (2S)-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-phenylpropanoate](/CAS/20200401/GIF/129397-81-5.gif)
129397-81-5
1 suppliers
inquiry

35661-40-6
425 suppliers
$5.00/5g

63-91-2
934 suppliers
$10.00/100G
82911-69-1
669 suppliers
$7.00/25g

35661-40-6
425 suppliers
$5.00/5g
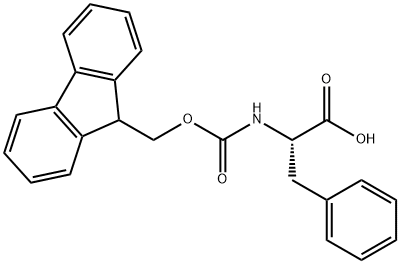
126727-04-6
27 suppliers
inquiry

35661-40-6
425 suppliers
$5.00/5g
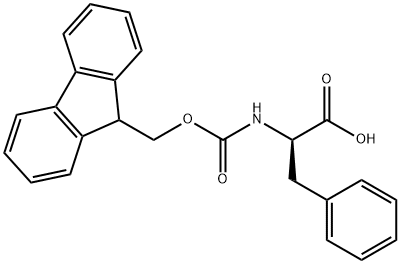
86123-10-6
340 suppliers
$6.00/5g