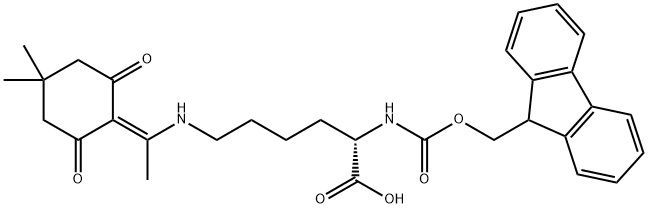
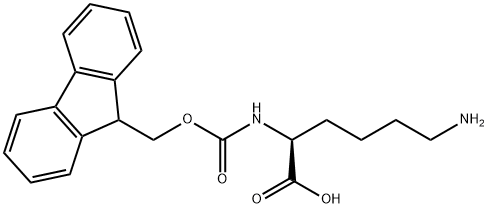
Fmoc-L-Lys(Dde)-OH synthesis
- Product Name:Fmoc-L-Lys(Dde)-OH
- CAS Number:150629-67-7
- Molecular formula:C31H36N2O6
- Molecular Weight:532.63
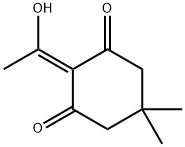
94142-97-9
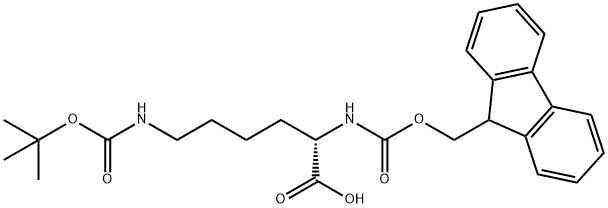
71989-26-9

150629-67-7
GENERAL STEPS: N-α-Fmoc-N-ε-Boc-L-lysine (5.66 g, 12.1 mmol) was dissolved in 4 M HCl/dioxane (120 mL) and stirred at room temperature for 2 h to remove the tert-butoxycarbonyl protecting group. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure. The resulting residue was dissolved in ethanol (60 mL) followed by the addition of 2-(1-hydroxyethylidene)-5,5-dimethylcyclohexane-1,3-dione (3.36 g, 18.4 mmol) and N,N-diisopropylethylamine (6.2 mL, 35.6 mmol). The reaction mixture was heated to reflux for 17 hours. After cooling to room temperature, the solvent was removed by concentration under reduced pressure. The residue was dissolved in ethyl acetate (300 mL), washed sequentially with 1 M HCl (100 mL) and saturated saline (100 mL), and the organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel column chromatography (0.5%-3% methanol/dichloromethane gradient elution) to afford N-fluorenylmethoxycarbonyl-N'-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl]-L-lysine (2.64 g, 41% yield) as a white solid. The spectral data of the product were consistent with those reported in the literature.1H NMR (500 MHz, CDCl3): δ 13.31 (br s, 1H), 7.75 (d, J = 7.8 Hz, 2H), 7.59 (t, J = 7.8 Hz, 2H), 7.38 (t, J = 7.8 Hz, 2H), 7.31-7.28 (m, 2H), 5.73 (d, J = 8.0 Hz, 1H), 4.48-4.45 (m, 1H), 4.37 (d, J = 7.1 Hz, 2H), 4.20 (t, J = 7.1 Hz, 1H), 3.43-3.40 (m, 2H), 2.55 (s, 3H), 2.36 (s, 4H), 2.00-1.50 (m, 6H), 1.01 (s, 6H). HR-MS (m/z, FAB): calculated C31H37N2O6 ([M+H]+), 533.2652; measured, 533.2643.

94142-97-9
107 suppliers
$22.00/1g

71989-26-9
504 suppliers
$5.00/1g

150629-67-7
232 suppliers
$6.00/100mg
Yield:150629-67-7 41%
Reaction Conditions:
Stage #1:Fmoc-Lys(tert-butoxycarbonyl) with hydrogenchloride in 1,4-dioxane at 20; for 2 h;
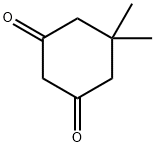
Stage #2:2-(1-hydroxyethylidene)-5,5-dimethylcyclohexane-1,3-dione with N-ethyl-N,N-diisopropylamine in ethanol for 17 h;Reflux;
Steps:
4.1.4. Synthesis of Fmoc-Lys(Dde)-OH
Fmoc-Lys(Boc)-OH (5.66 g, 12.1 mmol) was dissolved in 4 M HCl/dioxane (120 mL), and stirred at room temperature for 2 h to remove the side-chain Boc group. The solvent was removed under reduced pressure. The resulting residue was dissolved in EtOH(60 mL), and then 2-acetyldimedone (3.36 g, 18.4 mmol) and DIPEA (6.2 mL, 35.6 mmol) were added. The reaction mixture was refluxed for 17 h. After cooling to room temperature, the solvent was removed under reduced pressure. The residue was dissolved in AcOEt (300 mL) and washed with 1 M HCl (100 mL) and brine (100 mL), dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography(0.5%-3% MeOH/DCM) to give Fmoc-Lys(Dde)-OH (2.64 g,41%) as a white solid. Spectroscopic data are identical to the published data.34 1H NMR (500 MHz, CDCl3): d 13.31 (brs, 1H), 7.75(d, J = 7.8 Hz, 2H), 7.59 (t, J = 7.8 Hz, 2H), 7.38 (t, J = 7.8 Hz, 2H),7.31-7.28 (m, 2H), 5.73 (d, J = 8.0 Hz, 1H), 4.48-4.45 (m, 1H),4.37 (d, J = 7.1 Hz, 2H), 4.20 (t, J = 7.1 Hz, 1H), 3.43-3.40 (m, 2H),2.55 (s, 3H), 2.36 (s, 4H), 2.00-1.50 (m, 6H), 1.01 (s, 6H). HR-MS(m/z, FAB): calcd for C31H37N2O6 ([M + H]+), 533.2652; found,533.2643.
References:
Amano, Yuichi;Umezawa, Naoki;Sato, Shin;Watanabe, Hisami;Umehara, Takashi;Higuchi, Tsunehiko [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 3,p. 1227 - 1234]
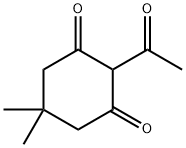
1755-15-3
114 suppliers
$13.00/250mg
105047-45-8
239 suppliers
$8.00/1g

150629-67-7
232 suppliers
$6.00/100mg
126-81-8
424 suppliers
$5.00/10g

150629-67-7
232 suppliers
$6.00/100mg

94142-97-9
107 suppliers
$22.00/1g

105047-45-8
239 suppliers
$8.00/1g

150629-67-7
232 suppliers
$6.00/100mg