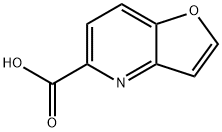
furo[3,2-b]pyridine-5-carboxylic acid synthesis
- Product Name:furo[3,2-b]pyridine-5-carboxylic acid
- CAS Number:56473-91-7
- Molecular formula:C8H5NO3
- Molecular Weight:163.13
![Furo[3,2-b]pyridine-5-carbonitrile(9CI)](/CAS/GIF/182691-67-4.gif)
182691-67-4
5 suppliers
inquiry
![furo[3,2-b]pyridine-5-carboxylic acid](/CAS/GIF/56473-91-7.gif)
56473-91-7
20 suppliers
inquiry
Yield:56473-91-7 50%
Reaction Conditions:
Stage #1: 5-cyanofuro<3,2-b>pyridinewith water;potassium hydroxide in ethanol; for 4 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=5-6;
Steps:
37B
To a solution of compound B-137 (450 mg, 3 mmol) in ethanol (10 mL) and water (2.5 mL) was added potassium hydroxide (1.2 g, 20 mmol). The mixture was heated at reflux for 4 hours. After evaporation to remove ethanol, the mixture was washed with ethyl acetate. The aqueous phase was adjusted to pH 5-6 with IN hydrochloric acid and extracted with DCM (15 mL χ 3). The combined organic layers were washed with water and brine, dried over anhydrous sodium sulfate and concentrated in vacuo to give crude compound B-138 (254 mg, 50% yield): i-NMR (CD3OD, 400 MHz): δ 8.22 (d, J=4.4 Ηζ, ΙΗ), δ 8.17 (d, J=8.4 Ηζ,ΙΗ), 8.07 (d, J=8.4 Hz, 2H), 7.12 (d, J=1.6
References:
WO2016/100184,2016,A1 Location in patent:Paragraph 00339-00340
![furo[3,2-b]pyridine](/CAS/GIF/272-62-8.gif)
272-62-8
89 suppliers
$36.00/100mg
![furo[3,2-b]pyridine-5-carboxylic acid](/CAS/GIF/56473-91-7.gif)
56473-91-7
20 suppliers
inquiry
![Furo[3,2-b]pyridine,4-oxide(9CI)](/CAS/GIF/181526-18-1.gif)
181526-18-1
21 suppliers
inquiry
![furo[3,2-b]pyridine-5-carboxylic acid](/CAS/GIF/56473-91-7.gif)
56473-91-7
20 suppliers
inquiry