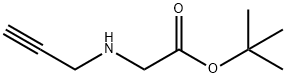
Glycine, N-2-propyn-1-yl-, 1,1-dimethylethyl ester synthesis
- Product Name:Glycine, N-2-propyn-1-yl-, 1,1-dimethylethyl ester
- CAS Number:66937-72-2
- Molecular formula:C9H15NO2
- Molecular Weight:169.22
Yield:66937-72-2 63%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20; for 20 h;
Steps:
1.1 Step 1:
Preparation of Compound (A5)
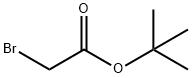
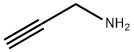
Into a 500 mL eggplant type flask, propargylamine (8.81 g, 160 mmol), N,N-dimethylformamide (112 mL) and potassium carbonate (18.5 g, 134 mmol) were charged in this order, and then adjusted to a temperature of 0° C., followed by dropwise addition of a solution wherein t-butyl bromoacetate (21.9 g, 112 mmol) was dissolved in N,N-dimethylformamide (80 mL) for about 1 hour while stirring.
After completion of the dropwise addition, the reaction solution was adjusted to room temperature, and then stirred for 20 hours.
Thereafter, the solid material was removed by filtration, and then 1 L of ethyl acetate was added to the filtrate, followed by washing four times with water and once with 300 mL of a saturated sodium chloride aqueous solution.
Then, the organic phase was dried by magnesium sulfate, and then the solvent was removed by evaporation under reduced pressure.
Finally, the residual oil material was subjected to distillation under reduced pressure under 0.6 Torr at 70° C., thereby to obtain a colorless liquid of t-butyl N-propargylamino acetate (compound (A5)).
The total amount of yield was 12.0 g, and the yield rate was 63%.
References:
US8901353,2014,B2 Location in patent:Paragraph 0189