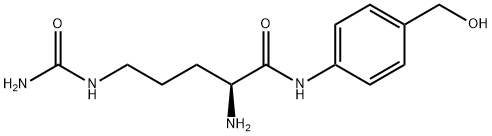
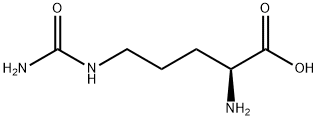
H-Cit-PABA synthesis
- Product Name:H-Cit-PABA
- CAS Number:1037794-22-1
- Molecular formula:C13H20N4O3
- Molecular Weight:280.32
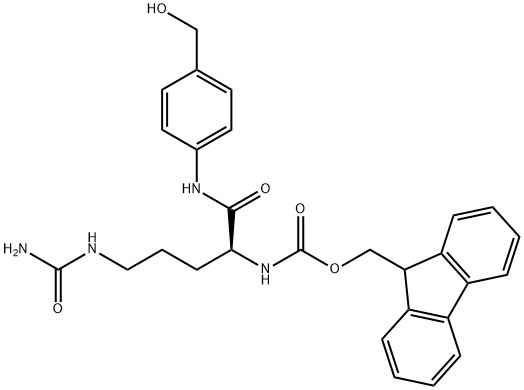
870487-04-0
36 suppliers
$111.00/1G

1037794-22-1
14 suppliers
inquiry
Yield:1037794-22-1 94%
Reaction Conditions:
with piperidine in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
13.2 Step 2 : Preparation of (S)- 1 -( 1 -(4-(hydroxymethyl)phenyl amino)- 1 -oxo-5 - ureidopentan-2-ylcarbamoyl)cyclobutanecarboxylic acid 10b
To a stirred solution of lOe (4.2 g, 8.3 mmol) in dry DMF (20 ml) was added piperidine (1.65 mL, 17 mmol, 2 eq) dropwise at r.t. The mixture was stirred at r.t. for 30 min, and solid precipitate formed. Dry DCM (50 mL) was added, and the mixture became transparent immediately. The mixture was stirred at r.t. for another 30 min, and LCMS showed lOe was consumed. It was concentrated to dryness under reduced pressure (make sure no piperidine remained), and the residue was partitioned between EtOAc and H20 (50 mL / 20 mL). Aqueous phase was washed with EtOAc (50 mL x 2) and concentrated to give (S)-2-amino-N-(4-(hydroxymethyl)phenyl)-5-ureidopentanamide lOd as an oily residual (2.2 g, 94%) (contained small amount of DMF).
References:
WO2016/90038,2016,A1 Location in patent:Page/Page column 124-125
![Carbamic acid, N-[(1S)-4-[(aminocarbonyl)amino]-1-[[[4-(hydroxymethyl)phenyl]amino]carbonyl]butyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1037793-80-8.gif)
1037793-80-8
1 suppliers
inquiry

1037794-22-1
14 suppliers
inquiry
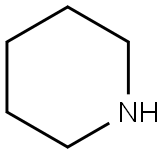
110-89-4
0 suppliers
$15.96/100ML

870487-04-0
36 suppliers
$111.00/1G

1037794-22-1
14 suppliers
inquiry
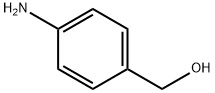
623-04-1
366 suppliers
$6.00/1g

1037794-22-1
14 suppliers
inquiry
372-75-8
791 suppliers
$6.00/10g

1037794-22-1
14 suppliers
inquiry