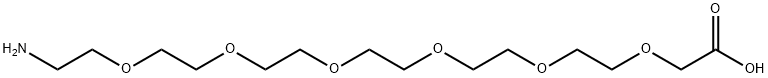
H2N-PEG6-CH2COOH synthesis
- Product Name:H2N-PEG6-CH2COOH
- CAS Number:1104083-46-6
- Molecular formula:C14H29NO8
- Molecular Weight:339.38

880129-82-8
26 suppliers
inquiry

1104083-46-6
19 suppliers
inquiry
Yield:1104083-46-6 99%
Reaction Conditions:
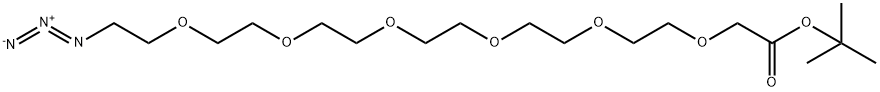
Stage #1: 2-((17-azido-3,6,9,12,15-pentaoxaheptadec-1-yl)oxy)acetic acidwith triphenylphosphine in tetrahydrofuran at 0 - 20; for 24 h;
Stage #2: with water in tetrahydrofuran at 20; for 24 h;
Steps:
6 1 -(4-((2,5-Dioxo-3-(((6S)-7-oxo-6-(5-oxopyrrolidine-2-carboxamido)-7- (phenylamino)heptyl)thio)pyrrolidin-1 -yl)methyl)cyclohexyl)-1 -oxo- 5,8,11 ,14,17,20-hexaoxa-2-azadocosan-22-oic acid [26]
Azide [24] (49 mg, 0.10 mmol) is dissolved in dry THF (10 mL) at 0 °C. Triphenyl phosphine (53 mg, 0.2 mmol) is added and the mixture stirred for 24 h at room temperature. H2O (5 mL) is then added to hydrolyse the intermediate phosphorus adducts and the solution stirred for another 24 h at room temperature. THF is evaporated and the solid residue suspended in water (10 mL). The insoluble salts are filtered, and the filtrate washed with toluene (5 x 5 mL) and evaporated to yield 20-amino-3,6,9, 12, 15, 18-hexaoxaicosanoic acid (46 mg, 0.1 mmol, 99%) as pale yellow oil, which is used in the next step without further purification.1H NMR (400 MHz, CDsOD) δ = 12.0 (bs, 1 H); 4.26 (s, 2H); 3.92-3.50 (m, 22H), 3.03-3.07 (m, 2H), 1 .81 (bs, 2H); MS: m/z 340 [M+H]+. 4-((2,5-Dioxo-3-(((6S)-7-oxo-6-(5- oxopyrrolidine-2-carboxamido)-7-(phenylamino)heptyl)thio)-pyrrolidin-1 - yl)methyl)cyclohexane-1 -carboxylic acid, (80 mg, 0.1 1 mmol), in dry CH2CI2 (5 mL), is added, at room temperature to a solution containing dicyclohexyl- carbodiimide (0.1 1 mmol) and N-hydroxysuccinimide (0.1 mmol), in dry CH2CI2 and the mixture stirred at room temperature for 3 hours. The white solid is filtered with dichloromethane to remove the dicyclohexylurea, the organic phase is washed with HCI 0.1 N and H2O, then dried over dry sodium sulfate and the solvent removed under reduced pressure. The resulting residue is purified by flash column chromatography to give the activated acid as a white waxy material (yield 90%) that is dissolved into dimethoxyethane (5 mL) and treated with 20-amino- 3,6,9, 12, 15, 18-hexaoxaicosanoic acid (46 mg, 0.1 mmol) dissolved in a mixture of tetrahydrofuran and aqueous sodium bicarbonate (15 mg, 0.15 mmol in 2 mL of water). The reaction is stirred at room temperature for 16 hours. A solution of citric acid 15% in water (2.5 mL) is added and the mixture extracted with 10% isopropyl alcohol in ethyl acetate (2 x 5 mL). The solvent is removed by rotatory evaporation. After addition of diethyl ether and irradiation with ultrasounds, the formation of a solid is obtained. Filtration followed by washing with diethyl ether gave [26] as a white solid, 65 mg (70%). MS: m/z 918 [M-H]-.
References:
WO2018/178060,2018,A1 Location in patent:Page/Page column 76-78

86770-69-6
91 suppliers
inquiry

1104083-46-6
19 suppliers
inquiry

28746-78-3
1 suppliers
inquiry

1104083-46-6
19 suppliers
inquiry
297162-49-3
38 suppliers
inquiry

1104083-46-6
19 suppliers
inquiry
42749-28-0
59 suppliers
$183.34/0.5g

1104083-46-6
19 suppliers
inquiry