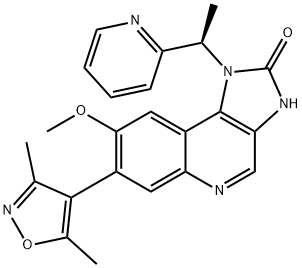
I-BET151 (GSK1210151A) synthesis
- Product Name:I-BET151 (GSK1210151A)
- CAS Number:1300031-49-5
- Molecular formula:C23H21N5O3
- Molecular Weight:415.44
![3-QuinolinecarboxaMide, 7-(3,5-diMethyl-4-isoxazolyl)-6-Methoxy-4-[[(1R)-1-(2-pyridinyl)ethyl]aMino]-](/CAS/20150408/GIF/1300737-69-2.gif)
1300737-69-2

1300031-49-5
7-(3,5-dimethylisoxazol-4-yl)-6-methoxy-4-(((R)-1-(pyridin-2-yl)ethyl)amino-3-quinolinecarboxamide (45 g, 102 mmol) was used as a starting material, which was dissolved in methanol (500 mL), followed by the addition of potassium hydroxide (7.47 g, 133 mmol). The reaction mixture was placed in an ice bath and stirred, then iodobenzene diacetate (39.6 g, 123 mmol) was added in batches over 20 minutes and stirring was continued for 1 hour. Upon completion of the reaction, the solvent was removed by vacuum evaporation, the residue was diluted with water (1 L), and the gelatinous suspension formed was extracted with dichloromethane (2 × 300 mL). The organic phases were combined, dried with sodium sulfate, and directly upsampled onto a silica column (750 g), which was eluted using a methanol/dichloromethane gradient (0-10%) of 2 M ammonia. The eluate was concentrated in vacuum to afford the target product 7-(3,5-dimethylisoxazol-4-yl)-8-methoxy-1-((R)-1-(pyridin-2-yl)ethyl)-1H-imidazo[4,5-c]quinolin-2(3H)-one (32.7 g, 77% yield) as a beige solid. The product was structurally confirmed by 1H-NMR (600 MHz, DMSO-d6), 13C NMR (151 MHz, DMSO-d6), mass spectrometry (MS) and high resolution mass spectrometry (HRMS).
![3-QuinolinecarboxaMide, 7-(3,5-diMethyl-4-isoxazolyl)-6-Methoxy-4-[[(1R)-1-(2-pyridinyl)ethyl]aMino]-](/CAS/20150408/GIF/1300737-69-2.gif)
1300737-69-2
2 suppliers
inquiry

1300031-49-5
140 suppliers
$35.00/1mg
Yield: 77%
Reaction Conditions:
with [bis(acetoxy)iodo]benzene;potassium hydroxide in methanol for 1.33333 h;Cooling with ice;Hofmann rearrangement;
Steps:
7-(3,5-dimethyl-4-isoxazolyl)-8-(methyloxy)-1-[(1R)-1-(2-pyridinyl)ethyl]-1,3-dihydro-2-Himidazo[4,5c]quinolin-2-one (I-BET151)
7-(3,5-Dimethyl-4-isoxazolyl)-6-(methyloxy)-4-{[(1R)-1-(2-pyridinyl)ethyl]amino}-3-quinolinecarboxamide (45 g, 102 mmol) was dissolved in methanol (500 mL) and potassium hydroxide (7.47 g, 133 mmol) was added. The mixture was stirred in an ice bath, then iodobenzene diacetate (39.6 g, 123 mmol) was added in small portions over 20 min and the mixture stirred for a further 1 h. The solvent was evaporated in vacuo, the residue diluted with water (1 L), and the resulting gummy suspension extracted with DCM (2 x 300 mL). The solvent was dried (sodium sulphate) and loaded directly onto a silica column (750 g), which was then eluted with a 2M ammonia in methanol / DCM gradient (0-10%). After evaporation of solvents in vacuo 7-(3,5-dimethyl-4-isoxazolyl)-8-(methyloxy)-1-[(1R)-1-(2-pyridinyl)ethyl]-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one (32.7 g, 77% yield) was obtained as a beige solid. 1H-NMR (600 MHz, DMSO-d6) d ppm 2.03 (d, J=6.5 Hz, 3 H), 2.04 (s, 3 H), 2.25 (s, 3 H), 3.46 (bs, 3 H), 6.26 (q, J=7.1 Hz, 1 H), 6.69 (bs, 1 H), 7.35 (dd, J=7.0, 5.1 Hz, 1 H), 7.41 (d, J=6.6 Hz, 1 H), 7.76-7.79 (m, 1 H), 7.79 (s, 1 H), 8.59-8.65 (m, 2 H), 11.77 (bs, 1H). 13C NMR (151 MHz, DMSO-d6) d ppm 10.3, 11.3, 17.6, 51.8, 55.5, 100.8, 112.0, 115.4, 120.2, 121.0, 122.7, 122.7, 128.1, 132.3, 132. 8, 137.6, 139.8, 149.3, 154.3, 154.5, 159.0, 159.2, 166.0. MS (Method HpH): MH+ 416, Rt 0.85 min. HRMS (m/z): [MH]+ calcd for C23H22N5O3 416.1723; found 416.1718.
References:
Mirguet, Olivier;Lamotte, Yann;Donche, Frédéric;Toum, Jérôme;Gellibert, Franoise;Bouillot, Anne;Gosmini, Romain;Nguyen, Van-Loc;Delannée, Delphine;Seal, Jonathan;Blandel, Florence;Boullay, Anne-Bénédicte;Boursier, Eric;Martin, Sandrine;Brusq, Jean-Marie;Krysa, Gael;Riou, Alizon;Tellier, Rémi;Costaz, Agns;Huet, Pascal;Dudit, Yann;Trottet, Lionel;Kirilovsky, Jorge;Nicodeme, Edwige [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 8,p. 2963 - 2967] Location in patent:supporting information; experimental part
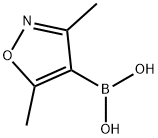
16114-47-9
252 suppliers
$6.00/250mg

1300031-49-5
140 suppliers
$35.00/1mg
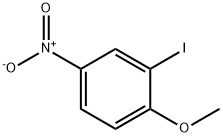
5399-03-1
136 suppliers
$8.00/1g

1300031-49-5
140 suppliers
$35.00/1mg
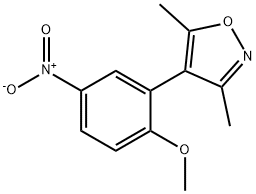
1300031-62-2
1 suppliers
inquiry

1300031-49-5
140 suppliers
$35.00/1mg
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry

1300031-49-5
140 suppliers
$35.00/1mg