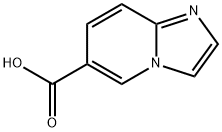
imidazo[1,2-a]pyridine-6-carboxylic acid synthesis
- Product Name:imidazo[1,2-a]pyridine-6-carboxylic acid
- CAS Number:139022-25-6
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
![IMidazo[1,2-a]pyridine-6-carboxylic acid, hydrochloride](/CAS/GIF/1314777-15-5.gif)
1314777-15-5
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
Step 2. Imidazo[1,2-a]pyridine-6-carboxylic acid hydrochloride (170 g) was dissolved in water (600 mL) and heated until completely dissolved to form a clarified solution. Subsequently, the pH was adjusted to 5-6 by slowly adding 2 M NaOH aqueous solution. the reaction mixture was cooled to 0 °C in an ice-water bath. The precipitate precipitated was collected by vacuum filtration, washed with ethanol and dried under vacuum to afford the target product imidazo[1,2-a]pyridine-6-carboxylic acid (107.2 g, 77% yield) as a light yellow powder. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6) and mass spectrometry (ESI+): 1H NMR δ 13.76-12.82 (br, 1H), 9.28 (s, 1H), 8.10 (s, 1H), 7.68 (s, 1H), 7.64-7.56 (m, 2H); MS (ESI+) m/z: 163 [M + H]+.
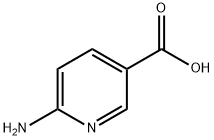
3167-49-5
399 suppliers
$8.00/5g
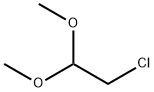
97-97-2
299 suppliers
$24.00/25mL
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg
Yield:139022-25-6 80.3 g
Reaction Conditions:
Stage #1: chloroacetaldehyde dimethyl acetalwith hydrogenchloride;water in ethanol at 25 - 85; for 1 h;
Stage #2: 6-aminonicotinic acid in ethanol;water at 85; for 24 h;
Steps:
1 Example 1: Preparation of imidazo[l,2-a]pyridine-6-carboxylic acid (IX):
A mixture of chloroacetaldehyde dimethylacetal (247.3 mL), 1M hydrochloric acid (800 mL) and ethanol (150 mL) was heated to 100 °C and stirred for 1 hour at the same temperature. The reaction mass was then cooled to 25 °C and its pH adjusted to 6.5-7.0 with solid sodium bicarbonate (68 g). 6-Amino nicotinic acid (100 g) was added to the above reaction mass in a portion wise manner at 25-30 °C. The reaction mass was slowly heated to 85 °C and stirred for 24 hours at the same temperature. The reaction mass was cooled to 0 °C and its pH adjusted to ~6 with a saturated solution of sodium bicarbonate. The resulting precipitate was filtered and dried at 40 °C for 4 hours to afford the title compound.Yield: 80.3 g
References:
WO2021/229605,2021,A1 Location in patent:Page/Page column 30
![IMidazo[1,2-a]pyridine-6-carboxylic acid, hydrochloride](/CAS/GIF/1314777-15-5.gif)
1314777-15-5
17 suppliers
$90.00/500mg
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg
![IMidazo[1,2-a]pyridine-6-carboxylic acid, ethyl ester](/CAS/GIF/158001-04-8.gif)
158001-04-8
44 suppliers
$86.00/100mg
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg

3167-49-5
399 suppliers
$8.00/5g
2032-35-1
487 suppliers
$5.00/10g
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg
![METHYL IMIDAZO[1,2-A]PYRIDINE-6-CARBOXYLATE](/CAS/GIF/136117-69-6.gif)
136117-69-6
125 suppliers
$32.00/500mg
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg