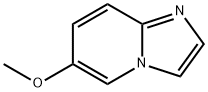
IMidazo[1,2-a]pyridine,6-Methoxy- synthesis
- Product Name:IMidazo[1,2-a]pyridine,6-Methoxy-
- CAS Number:955376-51-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

67-56-1
![6-IODOIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/426825-75-4.gif)
426825-75-4
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
General procedure for the synthesis of 6-methoxyimidazo[1,2-a]pyridine from methanol and 6-iodoimidazo[1,2-a]pyridine: In a solvent mixture of toluene (10 mL) and methanol (5 mL), 6-iodoimidazo[1,2-a]pyridine (1.0 g, 4.1 mmol) was added. After allowing to dissolve, copper(I) iodide (160 mg, 0.84 mmol), 1,10-phenanthroline (300 mg, 1.66 mmol) and cesium carbonate (3 g, 9 mmol) were added sequentially. The reaction mixture was stirred and reacted at 120 °C overnight. After completion of the reaction, it was cooled to room temperature and extracted by adding water and ethyl acetate. The organic layer was separated, washed sequentially with water and saturated saline and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (eluent ratio: hexane:ethyl acetate:methanol, 50:50:0 → 0:100:0 → 0:95:5, v/v) to afford the target compound 6-methoxyimidazo[1,2-a]pyridine (360 mg, 59% yield).1H-NMR (CDCl3) δ: 3.82 (3H s), 6.96 (1H, dd, J = 9.6, 2.3 Hz), 7.50 (1H, d, J = 9.6 Hz), 7.52 (1H, s), 7.57 (1H, s), 7.66 (1H, d, J = 2.3 Hz).
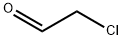
107-20-0
0 suppliers
$22.60/5ml
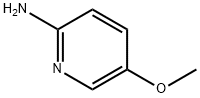
10167-97-2
226 suppliers
$18.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
46 suppliers
$55.00/100mg
Yield:955376-51-9 90%
Reaction Conditions:
with Sodium hydrogenocarbonate at 25 - 80; for 6 h;
Steps:
72.1 Step 1: 6-methoxyimidazo[1,2-a]pyridine
To a solution of 5-methoxypyridin-2-amine (5 g, 40.28 mmol) in EtOH (30 mL) was added NaHCCb (6.77 g, 80.55 mmol, 3.13 mL) and 2-chloroacetaldehyde (15.81 g, 80.55 mmol, 0.25 mL, 40% purity) at 25 °C. The mixture was stirred at reflux (80 °C) for 6 hrs. The reaction mixture was concentrated in vacuum. The residue was dissolved with water (30 mL), washed with EtOAc (50 mL c 2), then basified with saturated Na2CC>3 to pH = 11. The aqueous solution was extracted with EtOAc (50 mL c 3). The combined organic phase was dried over Na2S04 and concentrated in vacuum. The residue was purified by column chromatography (SiCh, Petroleum ether: EtOAc=15:l to 10:1) to afford the title compound (5.4 g, 90% yield) as a brown oil. [0758] NMR (400 MHz, CD3OD) d = 7.97 - 7.93 (m, 1H), 7.63 (s, 1H), 7.37 (s, 1H), 7.30 (d, J = 10.0 Hz, 1H), 6.98 - 6.88 (m, 1H), 3.70 (d, J = 1.8 Hz, 3H).
References:
WO2021/154842,2021,A1 Location in patent:Paragraph 0755-0758

67-56-1
790 suppliers
$9.00/25ml
![6-IODOIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/426825-75-4.gif)
426825-75-4
96 suppliers
$28.00/250mg
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
46 suppliers
$55.00/100mg
6602-32-0
421 suppliers
$10.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
46 suppliers
$55.00/100mg
24100-18-3
253 suppliers
$6.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
46 suppliers
$55.00/100mg
76066-07-4
99 suppliers
$44.00/250mg
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
46 suppliers
$55.00/100mg