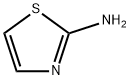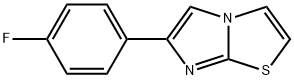
IMidazo[2,1-b]thiazole, 6-(4-fluorophenyl)- synthesis
- Product Name:IMidazo[2,1-b]thiazole, 6-(4-fluorophenyl)-
- CAS Number:7025-29-8
- Molecular formula:C11H7FN2S
- Molecular Weight:218.25
Yield:7025-29-8 86%
Reaction Conditions:
Stage #1: 2-thiazolylamine;2-bromo-4'-fluoroacetophenone in ethanol; for 16 h;Heating / reflux;
Stage #2: with ammonia in ethanol;water;Alkaline conditions;
Steps:
2.1
To a mixture of 2-aminothiazole (H) (23.7 g, 0.23 mol) and 2-bromo-l-(4-fluorophenyl)-ethanone (I) (50 g, 0.23 mol) is added absolute ethanol (600 mL). The reactionis allowed to reflux with vigorous stirring for 16 hours. The reaction mixture is reduced to halfits original volume in vacuo. The remaining liquid is poured onto ice and the solution madebasic by the addition of ammonium hydroxide solution (30%). The resulting fine solid isfiltered and washed with water. The dark yellow solid so obtained is dried in a vacuum oven at50 °C to provide 6-(4-fluorophenyl)-imidazo[2,l-b]thiazole (J) (43.0 g, 86 %).ESMS[M+H]+ ='HNMR (300 MHz CDC13) 5 8 - 7.6 (m, 3H), 7.38 (bs, 1H), 7.08 (bs, 2H), 6.79 (bs, 1H); 13C75 MHz (NMR CDC13) 5: 163.2 (d, C-F, J= 244.7 Hz), 150.1,146.8,130.3 (C-C-C-C-F),
References:
WO2004/110990,2004,A2 Location in patent:Page 41
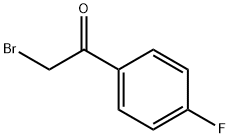
403-29-2
315 suppliers
$8.00/5g
![IMidazo[2,1-b]thiazole, 6-(4-fluorophenyl)-](/CAS/GIF/7025-29-8.gif)
7025-29-8
16 suppliers
inquiry
403-42-9
547 suppliers
$5.00/10g
![IMidazo[2,1-b]thiazole, 6-(4-fluorophenyl)-](/CAS/GIF/7025-29-8.gif)
7025-29-8
16 suppliers
inquiry