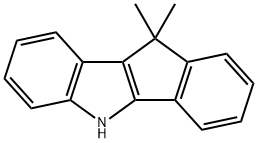
Indeno[1,2-b]indole, 5,10-dihydro-10,10-dimethyl- synthesis
- Product Name:Indeno[1,2-b]indole, 5,10-dihydro-10,10-dimethyl-
- CAS Number:133571-25-2
- Molecular formula:C17H15N
- Molecular Weight:233.31
Yield:133571-25-2 72%
Reaction Conditions:
with hydrogenchloride;glacial acetic acid in lithium hydroxide monohydrate; for 12 h;Reflux;
Steps:
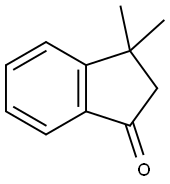
8.8-2 Synthesis of [Reaction Formula 8-2] [intermediate 8-b]
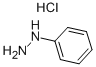
The [Reaction Scheme 8-1] obtained from [Intermediate 8-a] to show that the compound 60 g (375 mmol), phenylhydrazine hydrochloride 65 g (450 mmol) in 600 mL acetic acid, 30 mL of hydrochloric acid into a stirred 12 hr reflux . After the reaction was terminated with ethyl acetate and the organic layer was concentrated under reduced pressure and extracted with water and separated by column chromatography to give the compound 63 g (yield: 72%) represented by Formula 8-b].
References:
KR2015/111106,2015,A Location in patent:Paragraph 0409-0412