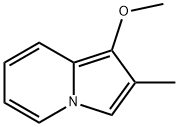
Indolizine, 1-methoxy-2-methyl- (9CI) synthesis
- Product Name:Indolizine, 1-methoxy-2-methyl- (9CI)
- CAS Number:610766-99-9
- Molecular formula:C10H11NO
- Molecular Weight:161.2
Yield:610766-99-9 77.5%
Reaction Conditions:
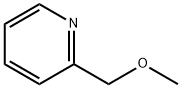
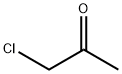
Stage #1: 2-(methoxymethyl)pyridine;chloroacetonewith potassium carbonate;lithium bromide in acetonitrile;Heating / reflux;Chichibabin Reaction;
Stage #2: with potassium carbonate in water;acetonitrile;
Steps:
III
The compounds of formula II, when R1 represents a radical -OCH3 or a radical -OCH2C6H5, are also prepared using the Tschitschibabin reaction according to the following synthesis schemes:; Preparation III Synthesis of 1-methoxy-2-methylindolizine This compound is obtained starting with 2-(methoxymethyl)pyridine and chloroacetone, using the Tschitschibabin reaction. The product is isolated in the form of a yellow oil which crystallizes in the freezer. Yield: 77.5% Mass spectrometry (ES+ mode): MH+=161.8
References:
US2005/203126,2005,A1 Location in patent:Page/Page column 10; 13