
Iodoacetonitrile synthesis
- Product Name:Iodoacetonitrile
- CAS Number:624-75-9
- Molecular formula:C2H2IN
- Molecular Weight:166.95

590-17-0

624-75-9
Step 2. The method followed the synthesis of literature S5: 2,6-piperidinedimethanol (3.80 g, 26.21 mmol), iodoacetonitrile (5.24 g, 31.45 mmol), triethylamine (Et3N, 4.38 mL, 31.45 mmol) and anhydrous N,N-dimethylformamide (DMF, 20 mL) were added to the reaction system. Upon completion of the reaction, the product was purified by fast column chromatography using a solvent mixture of dichloromethane (CH2Cl2) and methanol (CH3OH) (9:1, v/v) as eluent to afford iodoacetonitrile as a straw-colored oily substance (2.5 g, 53% yield). the molecular ion peak m/z (M + H)+ was determined by FAB MS to be 185. iodoacetonitrile was prepared using the Finkelstein reaction: Bromoacetonitrile (BrCH2CN, 3.77 g, 0.0314 mmol) was added dropwise to an acetone solution of sodium iodide (NaI, 4.71 g, 0.031 mmol) under stirring. A precipitate of sodium bromide (NaBr) was produced within a few minutes of the reaction, indicating that a halogen exchange reaction had occurred. The sodium bromide was removed by filtration and the acetone was subsequently evaporated under vacuum to give the crude product iodoacetonitrile (5.24 g, 100% yield).

590-17-0
337 suppliers
$5.00/5g

624-75-9
159 suppliers
$12.00/1g
Yield:624-75-9 100%
Reaction Conditions:
with sodium iodide in acetone
Steps:
7.2
Step 2. The method follows that of S5; 2, 6-piperidinedimethanol (3.80 g, 26.21 mmol), iodoacetonitrile (5.24 g, 31.45 mmol), Et3N (4. 38 mL, 31.45 mmol) and dry DMF (20 mL). Purification by flash chromatography using CH2CI2/CH30H (9: 1) as eluent afforded the title compound as a straw-coloured oil (2.5 g, 53 %). FAB MS, m/z (M+H) + 185. The iodoacetonitrile was prepared by the use of the Finkelstein reaction. BrCH2CN (3.77 g, 0.0314 mmol) was added dropwise to a stirred solution of Nal (4.71 g, 0.031 mmol) in acetone. Precipitation of NaBr occurred within a few minutes and indicated that exchange of the halides had taken place. Sodium bromide was filtered off, and the acetone was removed in vacuo. Crude yield (5.24 g, 100 %).
References:
SCHOOL OF PHARMACY, UNIVERSITY OF LONDON WO2005/61453, 2005, A1 Location in patent:Page/Page column 15

107-14-2
296 suppliers
$15.00/25g

624-75-9
159 suppliers
$12.00/1g
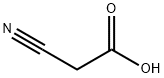
372-09-8
368 suppliers
$20.00/25g

624-75-9
159 suppliers
$12.00/1g
![[methyl(phenyl)amino]acetonitrile](/CAS/GIF/16728-83-9.gif)
16728-83-9
4 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g

624-75-9
159 suppliers
$12.00/1g
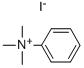
98-04-4
85 suppliers
$38.00/25g
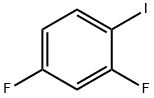
2265-93-2
234 suppliers
$8.00/5g

75-05-8
1049 suppliers
$10.00/10g

624-75-9
159 suppliers
$12.00/1g
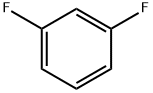
372-18-9
424 suppliers
$9.00/1g