
ISOXAZOL-5-YLACETONITRILE synthesis
- Product Name:ISOXAZOL-5-YLACETONITRILE
- CAS Number:854137-77-2
- Molecular formula:C5H4N2O
- Molecular Weight:108.1
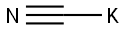
151-50-8
0 suppliers
$43.36/100g
69735-35-9
62 suppliers
$117.00/100mg

854137-77-2
18 suppliers
inquiry
Yield:854137-77-2 55%
Reaction Conditions:
in water;dimethyl sulfoxide at 0 - 20; for 3 h;
Steps:
48A; D
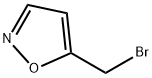
5-(Bromomethyl)isoxazole [P. DeShong, J.A. Cipollina, N.K. Lowmaster, J. Org. Chem. 1988, 53, 1356-1364] (42 g, 259 mmol) was dissolved in DMSO (420 mL). Water was added (145 mL), and the mixture was cooled with an ice-bath. Potassium cyanide (21.9 g, 337 mmol) was added, and the reaction was stirred at rt for 3 h. Subsequently, it was diluted with water (2 L), and the mixture was extracted three times with ethyl acetate (I L each). The combined organic layers were washed with brine, dried over sodium sulfate, and the solvent was evaporated. The crude product was purified by column chromatography on silica gel (eluent: cyclohexane/ethyl acetate 2:1) to yield15.5 g (55%) of the title product as an oil.1H-NMR (500 MHz, DMSOd6): δ = 4.45 (s, 2H), 6.52-6.54 (m, IH), 8.60 (d, IH).GC/MS (method 1): R, = 3.13 min; MS (ESIpos): m/z = 108 [M]+.
References:
WO2009/33581,2009,A1 Location in patent:Page/Page column 42; 85