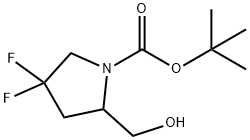
KQLZXWXCBWPDAD-UHFFFAOYSA-N synthesis
- Product Name:KQLZXWXCBWPDAD-UHFFFAOYSA-N
- CAS Number:317354-91-9
- Molecular formula:C10H17F2NO3
- Molecular Weight:237.24
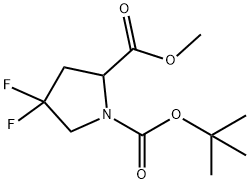
1000313-00-7
10 suppliers
inquiry

317354-91-9
9 suppliers
inquiry
Yield:317354-91-9 93%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;
Steps:
285 Synthesis of tert-butyl 4, 4-difluoro-2-(hydroxymethyl) pyrrolidine-1-carboxylate
To a stirred solution of 1-(tert-butyl) 2-methyl 4, 4-difluoropyrrolidine-1, 2- dicarboxylate (1.2 g, 4.52 mmol) in dry THF (12 mL) under an argon atmosphere was added lithium aluminum hydride (172 mg, 4.52 mmol) at 0 oC. The reaction mixture was stirred for 30 min. After consumption of starting material (monitored by TLC), the reaction mixture was filtered and washed with EtOAc (100 mL). The filtrate was concentrated in vacuo to obtain tert-butyl 4, 4-difluoro-2-(hydroxymethyl) pyrrolidine-1-carboxylate (1 g, 93%) as a colorless oil used in the next step without further purification. 1H-NMR (CDCl3 500 MHz): δ 4.20-4.13 (m, 1H), 3.82-3.65 (m, 2H), 2.53-2.49 (m, 1H), 2.16-2.12 (m, 1H), 1.63-1.61 (m, 2H), 1.45 (s, 9H), 1.29-1.26 (m, 1H); TLC: 20% EtOAc:hexanes (Rf: 0.3).
References:
WO2015/109109,2015,A1 Location in patent:Paragraph 1233
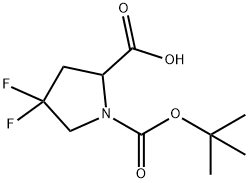
1000313-01-8
21 suppliers
$45.00/5mg

317354-91-9
9 suppliers
inquiry
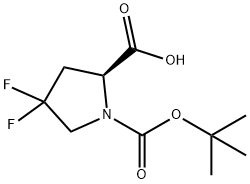
203866-15-3
173 suppliers
$15.00/250mg

317354-91-9
9 suppliers
inquiry