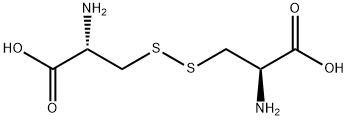
L-Cystine synthesis
- Product Name:L-Cystine
- CAS Number:56-89-3
- Molecular formula:C6H12N2O4S2
- Molecular Weight:240.3
70-18-8
1083 suppliers
$6.00/1g

56-89-3
922 suppliers
$5.00/10g
Yield:-
Reaction Conditions:
with mefenamic Acid in aq. phosphate buffer;ethanol;water;Electrolysis;
Steps:
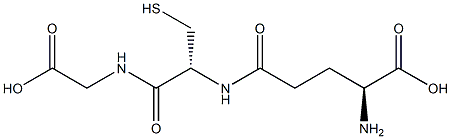
Synthesis of compounds 4 and 4′; Electroorganic synthesis of 4and 4′.-
General procedure: A mixture of a phosphate buffer (ca. 50 ml; c = 0.2 M,pH = 7.0) in water/ethanol (30:70 v/v) solution, containing mefenamic acid (1) (0.02 mmol) and glutathione (0.3 mmol) (3) orN-acetyl-L-cysteine (3′) was electrolyzed in a divided cell at 0.6 Vvs Ag/AgCl. The electrolysis was terminated when the currentdecreased by more than 95%. After having finished the electrolysis,the precipitated solid was collected by filtration and washed severaltimes with water. The products were characterized by infraredspectroscopy,7 mass spectroscopy, and melting point measurements.Characterization of product 4.-Mp > 238 °C (Dec.). IR(KBr):3030, 2917, 1622, 1586, 1488, 1382, 1297, 1194, 1091, 847, 778,and 675 cm-1. MS (EI): m/z (relativeintensity): 241 (M+., 8.1.1),169.1 (32.4), and 147.2 (100).
References:
Amani, Ameneh;Amooshahi, Parvaneh;Khazalpour, Sadegh [Journal of the Electrochemical Society,2020,vol. 167,# 4]
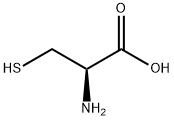
52-90-4
971 suppliers
$6.00/10g

56-89-3
922 suppliers
$5.00/10g
![2-amino-3-[(4-methoxyphenyl)methylsulfinyl]propanoic acid](/CAS/20180601/GIF/73243-09-1.gif)
![L-Alanine, 3-[[(acetylamino)methyl]sulfinyl]-](/CAS/20210305/GIF/75893-05-9.gif)
![2-Amino-3-[(4-methoxybenzyl)thio]propanoic acid](/CAS/GIF/2544-31-2.gif)