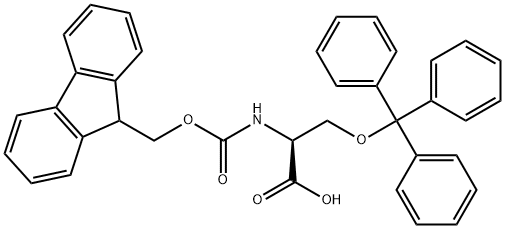
L-Serine,O-(triphenylmethyl)- synthesis
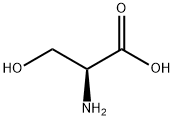
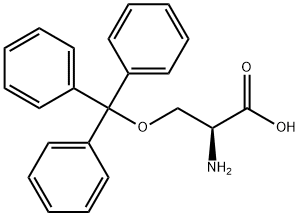
- Product Name:L-Serine,O-(triphenylmethyl)-
- CAS Number:25840-83-9
- Molecular formula:C22H21NO3
- Molecular Weight:347.41
Yield:25840-83-9 60.61%
Reaction Conditions:
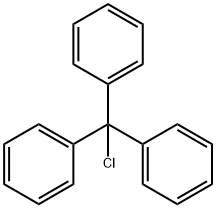
Stage #1: L-serin;trityl chloride;mercury dichloride in acetonitrile at 40;
Stage #2: with triethylamine in acetonitrile at 20; for 1 h;Product distribution / selectivity;
Steps:
15
Example 15:H-Ser(Tr)-OH (O-trityl serine) by selective 0- tritylation of serine.; 0.2 g of serine (1.9 mmol) require about 0.5 h to dissolve in a solution of 1.06 g of trityl chloride (3.8 mmol) and 0.52 g of HgCl2 (1.9 mmol) in 15 mL of EPO
References:
WO2007/9944,2007,A1 Location in patent:Page/Page column 30-31
111061-56-4
189 suppliers
$15.50/1G

25840-83-9
18 suppliers
inquiry