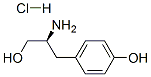
L-Tyrosinol hydrochloride synthesis
- Product Name:L-Tyrosinol hydrochloride
- CAS Number:87745-27-5
- Molecular formula:C9H14ClNO2
- Molecular Weight:203.67
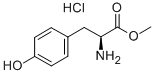
3417-91-2

87745-27-5
![2,5-Piperazinedione, 3,6-bis[(4-hydroxyphenyl)methyl]-, (3R,6S)-rel-](/CAS/20210305/GIF/10125-12-9.gif)
10125-12-9
General procedure for the synthesis of L-tyrosinol hydrochloride and compounds (CAS:10125-12-9) from L-tyrosine methyl ester hydrochloride: the reaction was carried out in a 0.5 L stainless steel autoclave with stirring at 500 rpm to assess the activity of the catalyst for the hydrogenation of R-phenylglycine methyl ester. First, 1 g of catalyst (20-40 mesh) was loaded into the reactor and subsequently purged five times with H2 to remove air. The catalyst was reduced at 1 MPa H2 pressure and 250 °C for 4 hours. After the autoclave was cooled to room temperature in H2 atmosphere, 1.5 g of R-phenylglycine methyl ester (R-p-m) diluted by 150 mL of ethanol (R-p-m/Cat = 1.5, w/w) was added. The standard reaction conditions were set at 5 MPa H2 pressure and 80 °C temperature for 10 h. The reaction was carried out in an autoclave. Upon completion of the reaction, the autoclave was cooled to room temperature in H2 atmosphere. Subsequently, the solid catalyst was separated by centrifugation. The product was purified by silica gel column chromatography using ethyl acetate/methanol (3/2, v/v) as eluent. Finally, the light yellow powdery product was obtained by rotary evaporation. The analysis of the reactants and products was carried out using a high performance liquid chromatograph (HPLC, Agilent 1260 Infinity) equipped with a UV detector and an Eclipse XDB-C18 column (150 × 4.6 mm, 5 μm particles), which in turn was used to calculate the conversion of R-phenylglycine methyl ester (X), yield (Y), and chemoselectivity for R-phenylglycine (S), where yield is the liquid chromatographic yield. The enantiomeric excess values (ee values) of the products were determined by HPLC equipped with a UV detector (wavelength 258 nm) and a chiral column (CHIRALPAK AY-H, 250 × 4.6 mm, 5 μm particle size).

3417-91-2
359 suppliers
$20.89/25g

87745-27-5
136 suppliers
$13.00/250mg
![2,5-Piperazinedione, 3,6-bis[(4-hydroxyphenyl)methyl]-, (3R,6S)-rel-](/CAS/20210305/GIF/10125-12-9.gif)
10125-12-9
0 suppliers
inquiry
Yield: 22.7 %Chromat.
Reaction Conditions:
with CuZn0.3Mg0.1AlO(x);hydrogen in ethanol at 80; under 37503.8 Torr; for 10 h;Autoclave;
Steps:
2.3 Testing of the Catalyst Activity
General procedure: The activity of catalyst for hydrogenation of R-phenylglycinemethyl ester was tested in a 0.5 L stainless steelautoclave under stirring at a speed of 500rpm. After 1g catalyst (20-40 mesh) was put in the reactor, the reactorwas swept with H2 five times to flush out air. Thenthe catalyst was reduced at 1MPa H2and 250 °C for 4h. After the autoclave was cooled in H2atmosphere to roomtemperature, 1.5g R-phenylglycine methyl esters (R-p-m)diluted in 150 mL ethanol was added (R-p-m/Cat = 1.5,wt.). The typical reaction conditions were at 5 MPa of H2 and 80 °C for 10 h. After the reaction was ended, theautoclave was cooled in H2atmosphere to room temperature.Then solid catalyst was separated by centrifugation.The product was purified by column chromatography on silica gel with ethyl acetate/methanol (3/2, v/v) as the eluent.Thus we obtained the light yellow powder product byrotary evaporation. Reactants and products were analyzedby High Performance Liquid Chromatograph (HPLC, Agilent1260 Infinity) equipped with an ultraviolet detector anda column (Eclipse XDB-C18, 150 × 4.6mm, 5mm particlesize), then the conversion of R-phenylglycine methyl ester(X), yield (Y) and chemoselectivity to R-phenylglycinol (S)were calculated [18, 19], in which the yield is the LC yield.And the ee value of products was determined by HPLCequipped with an ultraviolet detector (wavelength 258nm)and a chiral column (CHIRALPAK AY-H, 250 × 4.6mm,5m particle size) [19].
References:
Zhan, Bing;Zhang, Shuangshuang;Yu, Jun;Xiao, Xiuzheng;Guo, Xiaoming;Mao, Dongsen;Lu, Guanzhong [Catalysis Letters,2017,vol. 147,# 8,p. 2160 - 2166]
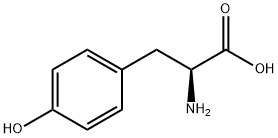
60-18-4
903 suppliers
$15.00/25g

87745-27-5
136 suppliers
$13.00/250mg