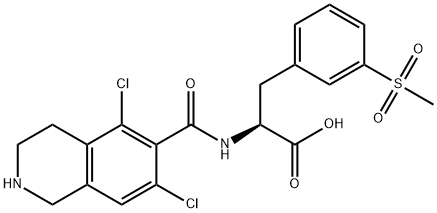
lifitegrast impurity C synthesis
- Product Name:lifitegrast impurity C
- CAS Number:851785-70-1
- Molecular formula:C20H20Cl2N2O5S
- Molecular Weight:471.35
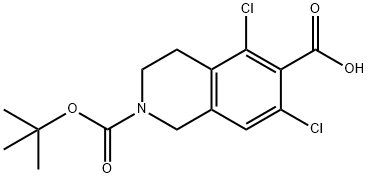
851784-82-2
180 suppliers
$11.00/250mg
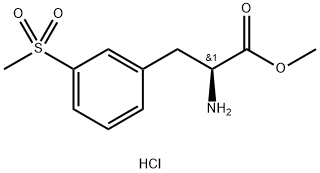
851785-21-2
115 suppliers
$18.00/100mg

851785-70-1
25 suppliers
inquiry
Yield:851785-70-1 91.8%
Reaction Conditions:
Stage #1: 2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid;methyl (2s)-2-amino-3-(3-(methylsulfonyl)phenyl)propionate hydrochloridewith N-ethyl-N,N-diisopropylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in N,N-dimethyl-formamide at 0 - 20;
Stage #2: with hydrogenchloride in water;
Steps:
15 Example 15: Synthesis of (S)-2-(5,7-dichloro-l,2,3i4-tetrahydroisoquinoline-6- carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid
2-(ieri-butoxycarbonyl)-5,7-dichloro- l,2,3,4-tetrahydroisoquinoline-6-carboxylic acid (20 g), HATU (27.5 g) was charged in DMF (60 ml) and diisopropyl ethylamine (22,2 g) was added. Reaction mass was cooled to 0-5°C and added methyl (S)-2-amino-3-(3- (m.ethylsuIfonyl)phenyl)propanoate hydrochloride (17.6g) lot wise at 0-5°C. Temperature of reaction mass was raised to room temperature. Stirred and reaction monitored by TLC. After completion of reaction, Water (100 ml) and MDC (100 ml) was added. Stirred and separated the layers. Organic layer washed with brine and 10% sodium carbonate solution. Organic layer distilled and charged 1 N HC1 (100 ml) to the residue. After complete hydrolysis pH of reaction mass was adjusted to 5.0-5.5 using sodium hydroxide solution. Reaction mass was filtered and solid so obtained was dried to get (S)-2-(5 ,7-dichloro- 1 ,2,3 ,4- tetrahydroisoquinoline-6-carb^ acid (25 g, (0185) 91.8% yield).
References:
WO2019/73325,2019,A1 Location in patent:Page/Page column 19
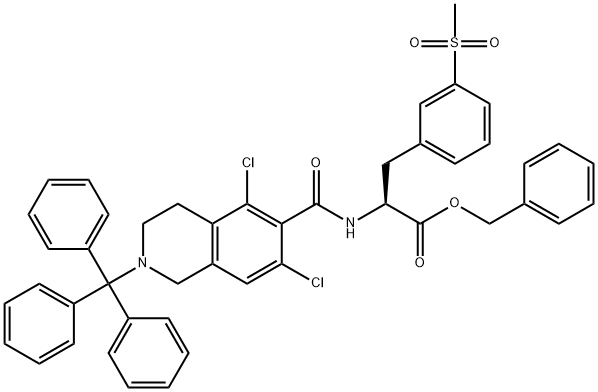
1194550-63-4
4 suppliers
inquiry

851785-70-1
25 suppliers
inquiry
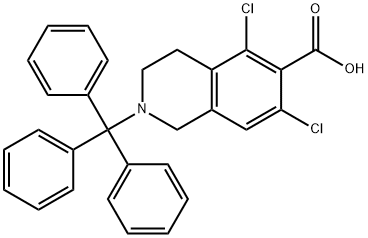
1194550-56-5
35 suppliers
inquiry

851785-70-1
25 suppliers
inquiry
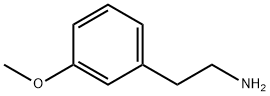
2039-67-0
243 suppliers
$17.46/5G

851785-70-1
25 suppliers
inquiry
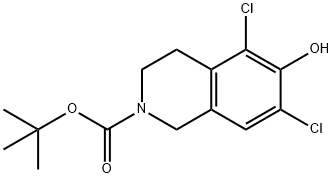
851784-76-4
16 suppliers
inquiry

851785-70-1
25 suppliers
inquiry