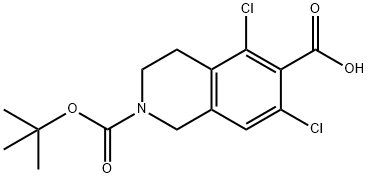
2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid synthesis
- Product Name:2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid
- CAS Number:851784-82-2
- Molecular formula:C15H17Cl2NO4
- Molecular Weight:346.21
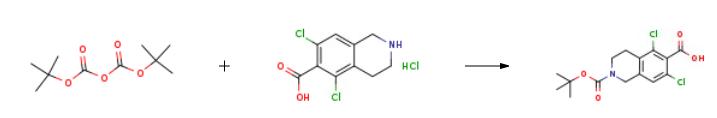
Boc-protection was used for the ring nitrogen in the intermediates 21 and 22.Compound 5 was deprotected with HC1 in dioxane to produce compound 23 in better than 97%yield. Boc-protection was introduced, using di-tert-butyl dicarbonate (1 .1 equivalent), and compound 21 was obtained in better than 95% yield. Compound 10 was coupled with compound 21 to obtain compound 22, using HATU and triethylamine in DMF. The product, compound 22, was obtained in quantitative yield, and greater than 90% purity. Deprotection with HC1 yielded the compound of Formula 12 in 97.4% yield.
851784-80-0
39 suppliers
inquiry

851784-82-2
180 suppliers
$11.00/250mg
Yield:851784-82-2 100%
Reaction Conditions:
with pyridine;lithium iodideHeating / reflux;
Steps:
1.b
b) A mixture of 1.1 (5 mmol) and 30mmol of LiI in 20mL of pyridine was reflux overnight. The solvent was removed and the residue was dissolved in EtOAc. The resulting solution was then washed with saturated aqueous NH4Cl and dried with anhydrous Na2S04. The solvent was removed and the residue was dried in vacuo to give a quantitative yield of compound 1.3. The crude product was carried on the next step without further purification. ESI-MS (m/z) : (M-tBu +1), 290.
References:
WO2005/44817,2005,A1 Location in patent:Page/Page column 77-79
851784-76-4
16 suppliers
inquiry

851784-82-2
180 suppliers
$11.00/250mg
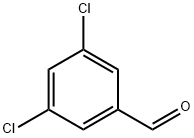
10203-08-4
314 suppliers
$8.00/5g

851784-82-2
180 suppliers
$11.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

851784-82-2
180 suppliers
$11.00/250mg
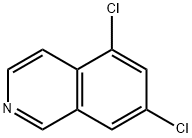
73075-58-8
18 suppliers
inquiry

851784-82-2
180 suppliers
$11.00/250mg