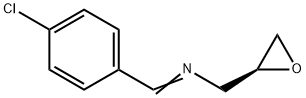
LinezolidImpurity36 synthesis
- Product Name:LinezolidImpurity36
- CAS Number:1345879-85-7
- Molecular formula:C10H10ClNO
- Molecular Weight:195.65
![(S)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol](/CAS/20180527/GIF/1345879-87-9.gif)
1345879-87-9
18 suppliers
inquiry

1345879-85-7
3 suppliers
inquiry
Yield:1345879-85-7 100%
Reaction Conditions:
with potassium carbonate in methanol; for 2 h;Product distribution / selectivity;
Steps:
31
EXAMPLE 31. Scale-up of synthesis of (S) (E, Z)-N-4-chlorobenzylidene-l- (oxiran-2-yl)methanamine (16). (S) (E,Z)-l-(4-Chlorobenzylideneamino)-3-chloropropan-2- ol (18) (163 g, 0.7 mol), reagent grade methanol (1.5 L) and anhydrous K2C03 (193 g, 1.4 mol) were added to a 3 L 3 neck round bottom flask with overhead stirrer. The reaction mixture was stirred vigorously. After 2 hrs GCMS showed complete conversion to the epoxide (small aliquot removed from the reaction, diluted with an equal volume of CH3CN, filtered through disposable pipette with a cotton plug). The colorless reaction was diluted with CH2CI2 (1 L), brine (800 ml) and water (200 ml). The aqueous layer was extracted with additional CH2CI2 (250 ml). The combined organic layers were washed with brine (4 x 200 ml), dried (MgS04) and evaporated (bath temp 45 °C) to a colorless oil (136g, quantitative); 1H NMR (500 MHz, DMSO-d6) δ 8.35 (s, 1H), 7.78 (d, 2H, J=8.2 Hz) ), 7.53 (d, 2H, J=8.2 Hz), 3.89 (ddd, 1H, J=13 Hz, J=3.5 Hz, J= 1.6 Hz), 3.55(ddd, 1H, J= 13 Hz, J= 6.1 Hz, J= 1.3 Hz), 3.25 (br ddd, 1H, J= 6.5 Hz, J=3.5Hz, J=2.8 Hz), 2.65 (dd, 1H, J= 5 Jz, J= 2.6 Hz), 2.77 (dd, 1H, J= 5Hz, J= 4.1 Hz); GCMS Retention time 8.98 min (m/e 194).
References:
WO2011/137222,2011,A1 Location in patent:Example 31
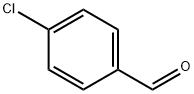
104-88-1
645 suppliers
$5.00/25g

1345879-85-7
3 suppliers
inquiry