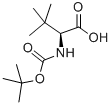
N-Boc-L-tert-Leucine synthesis
- Product Name:N-Boc-L-tert-Leucine
- CAS Number:62965-35-9
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
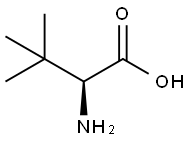
20859-02-3

24424-99-5

62965-35-9
Step A: Synthesis of N-Boc-L-tert-Leucine 1. add triethylamine (56mL, 0.4mol) to a methanol (150mL) suspension of L-tert-leucine (26.1g, 0.2mol) at 0°C. 2. slowly add di-tert-butyl dicarbonate (48g, 0.22mol) to a methanol (40mL) solution of N-Boc-L-tert-leucine. 2. a solution of di-tert-butyl dicarbonate (48 g, 0.22 mol) in methanol (40 mL) was added slowly to the above mixture, controlling the reaction temperature between 0 and 5°C. 3. The reaction mixture was stirred at room temperature overnight. 4. Upon completion of the reaction, the solvent was removed under vacuum. 5. The residue was dissolved in ethyl acetate (200 mL) and the organic layer was washed with 10% w/v aqueous citric acid (3 x 100 mL). 6. The organic layer was dried with anhydrous magnesium sulfate, filtered and the solvent was removed in vacuum to afford N-Boc-L-tert-leucine as a light yellow oil (48.9 g, yield >100% with residual solvent). Product characterization: 1H NMR (CDCl3): δ/ppm 9.20 (1H, bs, NH), 5.10 (1H, m, CH), 4.15 (1H, m, CH), 1.45 (9H, s, tert-butyl in Boc), 1.00 (9H, s, tert-butyl in tert-leucine).

20859-02-3
616 suppliers
$9.00/5G

24424-99-5
868 suppliers
$13.50/25G

62965-35-9
427 suppliers
$6.00/5g
Yield:62965-35-9 100%
Reaction Conditions:
with triethylamine in methanol at 0 - 5;
Steps:
1.A STEP A; 2(S)-tert-Butoxycarbonylamino-3,3-dimethyl-butyric Acid
STEP A: 2(S)-tert-Butoxycarbonylamino-3,3-dimethyl-butyric Acid To a suspension of L-tert-leucine (26.1 g, 0.2 mol) in methanol (150 ML) was added triethylamine (56 ML, 0.4 mol) and the mixture cooled to 0° C. To the mixture was added slowly a solution of di-tert-butyldicarbamate (48 g, 0.22 mol) in methanol (40 ML) such that an internal temperature of between 0 and 5° C. was maintained.. The reaction was allowed to stir overnight and the solvents were removed in vacuo.. The residue was dissolved in ethyl acetate (200 ML) and washed with 10% w/v aqueous citric acid solution (3*100 ML).. The organic layer was dried over MgSO4, filtered and the solvents removed in vacuo to give the title product as a pale yellow oil (48.9 g, >100%, residual solvent). 1H NMR (CDCl3): δ/ppm 9.20 (1H, bs), 5.10 (1H, m), 4.15 (1H, m), 1.45 (9H, s), 1.00 (9H, s).
References:
US6716878,2004,B1 Location in patent:Page column 14
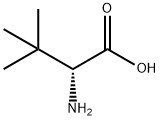
26782-71-8
362 suppliers
$12.50/1G

24424-99-5
868 suppliers
$13.50/25G

62965-35-9
427 suppliers
$6.00/5g
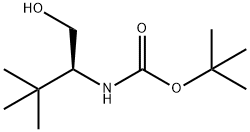
153645-26-2
63 suppliers
$75.00/1g

62965-35-9
427 suppliers
$6.00/5g

335627-81-1
2 suppliers
inquiry

62965-35-9
427 suppliers
$6.00/5g

20859-02-3
616 suppliers
$9.00/5G
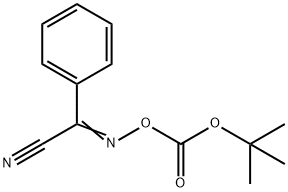
58632-95-4
271 suppliers
$7.00/5g

62965-35-9
427 suppliers
$6.00/5g