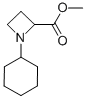
Methyl 1-cyclohexyl-2-azetidinecarboxylate synthesis
- Product Name:Methyl 1-cyclohexyl-2-azetidinecarboxylate
- CAS Number:18085-36-4
- Molecular formula:C11H19NO2
- Molecular Weight:0
Yield:18085-36-4 76%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 20; for 48 h;
Steps:
J.19A J. Synthesis of the Intermediate Azetidine 19 General Procedure
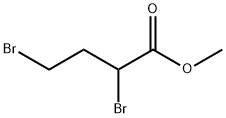
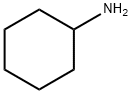
J. Synthesis of the Intermediate Azetidine 19 General Procedure [0163] To a solution of methyl 2,4-dibromobutanoate 17 (543 μA, 3.73 mmol) and the amine 18 (4.11 mmol) in acetonitrile (5 mL) was added under stirring ethyldiisopropyl amine (2.33 mL, 13.1 mmol). The reaction was stirred at room temperature for 2 days and concentrated under reduced pressure. Saturated aqueous sodium hydrogen carbonate solution was added and the reaction was extracted three times with diethyl ether. The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was chromatographed on silica using a gradient of a mixture of heptane and ethyl acetate (v:v 4:1) to a ethyl acetate to yield the azetidine 19. 19A. The reaction of methyl 2,4-dibromobutanoate 17 with cyclohexylamine 18A yielded methyl 1-(cyclohexyl)azetidine-2-carboxylate as a brown oil (76%). MS ISP (m/e): 198.2 (100) [(M+H)]+.
References:
US2013/217665,2013,A1 Location in patent:Paragraph 0163