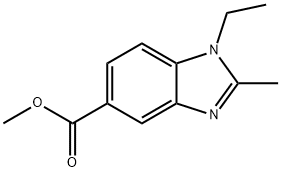
Methyl 1-ethyl-2-MethylbenziMidazole-5-carboxylate synthesis
- Product Name:Methyl 1-ethyl-2-MethylbenziMidazole-5-carboxylate
- CAS Number:306278-47-7
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25

67-56-1
790 suppliers
$9.00/25ml
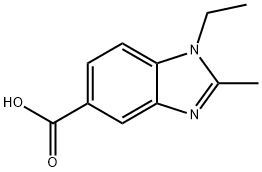
2788-73-0
17 suppliers
$75.00/250mg

306278-47-7
14 suppliers
$110.00/1g
Yield:-
Reaction Conditions:
Stage #1: methanol;1-ethyl-2-methyl-1H-benzoimidazole-5-carboxylic acidwith thionyl chloride at 20 - 70; for 24 h;
Stage #2: with sodium hydrogencarbonate in water;
Steps:
11.D
D. 1-Ethyl-2-methyl-1 H-benzoimidazole-5-carboxylic acid methyl ester; To a suspension of 1.1 g (5.4 mmol) of the title C compound, 1-ethyl-2-methyl-1 H- benzoimidazole-5-carboxylic acid in 25 mL of MeOH is added dropwise 0.7 g (5.9 mmol) of thionyl chloride and the resulting solution is stirred at 70° for 6 h, then at RT for 18 h. The solvent is removed under reduced pressure and 8% aqueous NaHC03 solution is added to the residue. The mixture is extracted with EtOAc and the organic phase is dried over anhydrous Na2SO4. The organic solution is concentrated until the product precipitated. The solid is collected by filtration, washed with EtOH and MTBE to give 1-ethyl-2-methyl-1 H- benzoimidazole-5-carboxylic acid methyl ester as a beige solid : mp = 118-121°C ; IR (KBr) 3430,1695 cm~';'H-NMR (CDCI3) 8 8.39 (s, 1H), 7.98 (dd, 1H), 7.32 (d, J = 8.45, 1H), 4.19 (q, 2H), 3.94 (s, 3H), 2.64 (s, 3H), 1.43 (t, 3H); [M+1] + = 219.
References:
WO2003/82841,2003,A1 Location in patent:Page/Page column 51