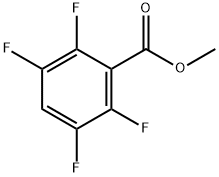
Methyl 2,3,5,6-tetrafluorobenzoate synthesis
- Product Name:Methyl 2,3,5,6-tetrafluorobenzoate
- CAS Number:4707-12-4
- Molecular formula:C8H4F4O2
- Molecular Weight:208.11
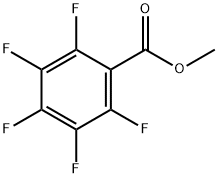
36629-42-2
163 suppliers
$15.00/5g

4707-12-4
17 suppliers
inquiry
Yield:4707-12-4 97%
Reaction Conditions:
with tris[2-phenylpyridinato-C2,N]iridium(III);N-ethyl-N,N-diisopropylamine in acetonitrile at 23; for 12 h;Sealed tube;Inert atmosphere;Photolysis;
Steps:
7.A [0174] Description of reaction shown in FIG. 43, Panel A:
In an NMR tube capped with NMR septa (Ace glass, part no. 9096-25) was charged tris(2-phenyl pyridinato-C 2, N) Iridium(III) (Ir(ppy)3) (0.250 mM, 1.00 mL in MeCN), methyl 2,3,4,5,6-pentafluorobenzoate (23.0 mg, 0.100 mmol) and DIPEA (35 μL, 0.200 mmol) were added, and the reaction was degassed via Ar bubbling for 10 min, at 0°C., to avoid evaporation of DIPEA and any other volatile starting materials, and then left under positive Ar pressure by removing the exit needle and then the argon supply needle. The tube was placed in a light bath (vide supra), and the lower portion of the tube was submerged under the water bath which was maintained at 23°C. The reaction was monitored by 19F NMR and GC-MS. After the complete consumption of starting material, the MeCN was removed via rotovap, and the residue was treated with deionized water (2 mL) and extracted with EtOAc (51 mL). The combined organic portions were dried with over MgSO4, filtered, and concentrated in vacuo. The crude material was purified by flash chromatography using hexane:EtOAc (0-50% EtOAc for 6 cv and ramped to 100% EtOAc for 6-26 cv and then held at 100% EtOAc 26-45 cv) on a 4 g silica column to afford of methyl 2,3,5,6-tetrafluorobenzoate in 97% yield (20.0 mg, 0.100 mmol) as a brown oil.
References:
US2020/399196,2020,A1 Location in patent:Paragraph 0173-0174
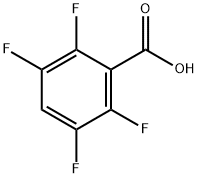
652-18-6
210 suppliers
$14.00/1g

4707-12-4
17 suppliers
inquiry
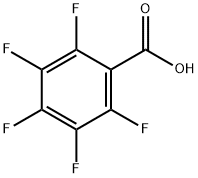
602-94-8
328 suppliers
$7.00/10g

4707-12-4
17 suppliers
inquiry

