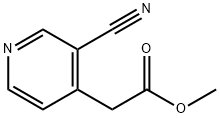
Methyl 2-(3-cyanopyridin-4-yl)acetate synthesis
- Product Name:Methyl 2-(3-cyanopyridin-4-yl)acetate
- CAS Number:124870-33-3
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
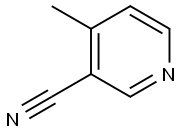
5444-01-9

616-38-6

124870-33-3
Preparation of the intermediate 2-(3-cyanopyridin-4-yl)acetic acid methyl ester (I-65a): in a dry reaction flask, 4-methylnicotinonitrile (2.52 g, 21.33 mmol) was dissolved in anhydrous THF (15 mL) and cooled to -78 °C. A THF solution of 1 M LiHMDS (45 mL, 44.794 mmol) was slowly added under nitrogen protection, kept at -78 °C and stirred for 1 hour. Subsequently, dimethyl carbonate (1.98 mL, 23.464 mmol) was added dropwise to the reaction system and stirring was continued at -78 °C for 1 h. The temperature was then slowly raised to 0 °C and stirred for 2 h. The reaction was carried out by TLC. The progress of the reaction was monitored by TLC (unfolding agent: hexane solution of 30% ethyl acetate). Upon completion of the reaction, the reaction was quenched with saturated NH4Cl solution and then extracted with ethyl acetate. The organic layers were combined, washed sequentially with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: hexane solution of 25% ethyl acetate) to give 530 mg of the target product in 14.10% yield. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 8.9 (s, 1H), 8.79 (d, 1H), 7.4 (d, 1H), 3.9 (s, 2H), 3.79 (s, 3H).

616-38-6
720 suppliers
$5.00/5 g

124870-33-3
39 suppliers
inquiry
Yield:124870-33-3 85%
Reaction Conditions:
Stage #1:3-cyano-4-methylpyridine with lithium hexamethyldisilazane in tetrahydrofuran at -78; for 1 h;
Stage #2:carbonic acid dimethyl ester in tetrahydrofuran at -78 - 0; for 3 h;
Steps:
1
2.52g of 4-methyl-nicotinonitrile was dissolved in 15 mL of anhydrous THF and dropwisely added with 45 mL of 1M LHMDS at-78 °C and stirred for about 1 hour. At the temperature the above mixture was dropwisely added with 1. 98 mL of dimethylcarbonate, stirred for about 1 hour. The mixture was then heated to 0 °C and stirred further for about 2 hours. The above mixture was added with 5 mL of saturated solution of ammonium chloride, diluted with 300 mL ethylacetate. Then, the organic solvent layer was washed with water, and saturated solution of sodium chloride, dried with anhydrous sodium sulfate and filtered. The filtrate was concentreated under reduced pressure and a silica gel column chromatography was performed on the resulting residue by using a mixed eluant of ethylacetate and hexane, wherein ethylacetate and hexane are mixed in 1: 3 volume ratio, and 3.21g (85%) of (3-cyano-pyridine-4-yl) -acetic acid methyl ester was obtained in colorless oil. H NMR (300 MHz, CDCIs) 6 8. 87 (s, 1H), 8. 75 (d, 1H, J=5. lHz), 7. 41 (d, 1H, J=5. 1Hz), 3.89 (s, 2H), 3.77 (s, 3H).
References:
SK CHEMICALS, CO., LTD. WO2005/63768, 2005, A1 Location in patent:Page/Page column 28

5444-01-9
233 suppliers
$16.00/1 g

616-38-6
720 suppliers
$5.00/5 g

124870-33-3
39 suppliers
inquiry