
Methyl 2-amino-5-fluoro-3-nitrobenzoate synthesis
- Product Name:Methyl 2-amino-5-fluoro-3-nitrobenzoate
- CAS Number:328547-11-1
- Molecular formula:C8H7FN2O4
- Molecular Weight:214.15

67-56-1
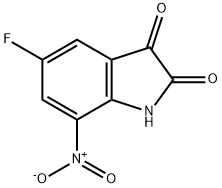
954571-39-2

328547-11-1
Step 1. 5-Fluoro-7-nitroindoline-2,3-dione (1.5 g, 7.4 mmol) was suspended in aqueous NaOH (5N, 14.0 mL) followed by addition of aqueous H2O2 (2.0 mL). The reaction mixture was stirred at room temperature for 5 h and then acidified with 2N HCl to pH=4.0. The resulting precipitate was filtered and dried in a vacuum oven to give a crude product which was used directly in the next step of the reaction. Step 2. The crude product obtained from Step 1 was dissolved in methanol (15.0 mL) and p-toluenesulfonic acid hydrate (1.33 g, 7.0 mmol) was added. The reaction mixture was refluxed for 36 hours. After cooling to room temperature, the reaction mixture was concentrated in vacuum to obtain the crude product. The crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (8:1, v/v) as eluent to give the yellow solid product methyl 2-amino-5-fluoro-3-nitrobenzoate (500 mg, 32% overall yield in two steps).

67-56-1
790 suppliers
$9.00/25ml

954571-39-2
20 suppliers
$45.00/25mg

328547-11-1
41 suppliers
inquiry
Yield:328547-11-1 30%
Reaction Conditions:
Stage #1: 5-fluoro-7-nitroindoline-2,3-dionewith dihydrogen peroxide;sodium hydroxide in water at 20; for 5 h;
Stage #2: methanolwith toluene-4-sulfonic acid for 36 h;Reflux;
Steps:
Methyl 2-amino-5-methyl-3-nitrobenzoate (94)
General procedure: Step 1.To a suspension of 91 (1.5 g, 7.4 mmol) in NaOH aq. solution (5 N, 14.0 mL) was added H2O2 aq. solution (2.0 mL). After stirred for another 5 h at room temperature, the reaction mixture acidified with 2 N conc. HCl untill pH = 4.0. The formed precipitate was filtered and dried in a vacuum oven to give a crude product for use next step without further purification.Step 2. To the solution of crude product of step 1 in methol (15.0 mL) was added p-toluenesulfonic acid hydrate (1.33 g, 7.0 mmol). The reaction mixture was refluxed for 36 h. After cooled to room temperature, the reaction mixture was concentrated under vacuum to give crude product. The crude product was chromatographed on silica gel using petroleum ether/ethyl acetate (8:1, v/v) as the eluent to give a yellow solid (500 mg, 32% for two steps).
References:
Chen, Xuxing;Huan, Xiajuan;Liu, Qiufeng;Wang, Yuqin;He, Qian;Tan, Cun;Chen, Yi;Ding, Jian;Xu, Yechun;Miao, Zehong;Yang, Chunhao [European Journal of Medicinal Chemistry,2018,vol. 145,p. 389 - 403] Location in patent:supporting information
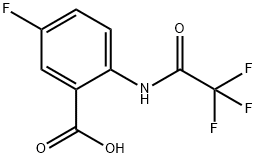
49579-59-1
4 suppliers
inquiry

328547-11-1
41 suppliers
inquiry
![5-Fluoro-3-nitro-2-[(2,2,2-trifluoroacetyl)amino]-benzoic Acid](/CAS/20210111/GIF/1266115-00-7.gif)
1266115-00-7
2 suppliers
inquiry

328547-11-1
41 suppliers
inquiry
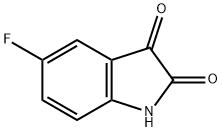
443-69-6
388 suppliers
$5.00/1g

328547-11-1
41 suppliers
inquiry