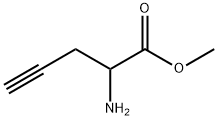
methyl 2-aminopent-4-ynoate synthesis
- Product Name:methyl 2-aminopent-4-ynoate
- CAS Number:70837-20-6
- Molecular formula:C6H9NO2
- Molecular Weight:127.14

67-56-1
782 suppliers
$9.00/25ml
50428-03-0
64 suppliers
$22.00/250mg

70837-20-6
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with thionyl chloride at 0; for 3 h;Heating / reflux;
Steps:
1.A
A) Methyl 2-(((4-methylphenyl)sulfonyl)amino)pent-3-ynoate 2.5 g of 2-aminobut-3-ynoic acid are suspended in 45 ml of methanol at 0° C. 1.8 ml of thionyl chloride are run in dropwise at this temperature and the mixture is then refluxed for 3 hours. The solution is concentrated and the residue is dried under reduced pressure. The latter is solubilized in 60 ml of acetonitrile followed by 5.4 ml of triethylamine and then 4.6 g of tosyl chloride are added. The mixture is stirred at ambient temperature for 19 hours and then at 50° C. for a further one hour. After concentration, the crude product is solubilized in dichloromethane and the organic phase is washed successively with a saturated aqueous solution of KHSO4 and then of K2CO3. The organic phase is dried over magnesium sulfate and then filtered and, finally, concentrated so as to obtain 5.18 g of the expected compound. 1H NMR: δ (ppm): 2.35: s: 3H, 2.45: up: 2H, 3.45: s: 3H, 3.9: rd: 1H; 7.35: d: 2H, 7.65: d: 2H, 8.4: d: 1H.
References:
US2008/176924,2008,A1 Location in patent:Page/Page column 6
![4-Pentynoic acid, 2-[(diphenylmethylene)amino]-, methyl ester](/CAS/20210305/GIF/167904-79-2.gif)
167904-79-2
0 suppliers
inquiry

70837-20-6
3 suppliers
inquiry
61172-60-9
0 suppliers
inquiry

70837-20-6
3 suppliers
inquiry