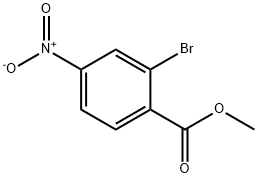
METHYL 2-BROMO-4-NITROBENZOATE synthesis
- Product Name:METHYL 2-BROMO-4-NITROBENZOATE
- CAS Number:100959-22-6
- Molecular formula:C8H6BrNO4
- Molecular Weight:260.04
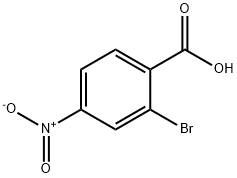
16426-64-5

74-88-4

100959-22-6
Example 54B Synthesis of methyl 2-bromo-4-nitrobenzoate: 2-bromo-4-nitrobenzoic acid (1.0 g, 4.06 mmol) and potassium carbonate (560 mg) were dissolved in N,N-dimethylformamide (5 mL), followed by the addition of iodomethane (500 μL, 8.03 mmol). The reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the mixture was poured into water (30 mL) and extracted with ether (3 x 10 mL). The combined ether layers were washed sequentially with water (1 × 10 mL) and brine (1 × 10 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to afford the target product methyl 2-bromo-4-nitrobenzoate (970 mg, 92% yield). The product was confirmed by 1H NMR (300 MHz, CDCl3): δ 8.52 (d, 1H, J=2.4 Hz), 8.21 (dd, 1H, J=2.0,8.5 Hz), 7.92 (d, 1H, J=8.8 Hz), 3.99 (s, 3H); the mass spectrum (ESI) showed m/z=259 (M-H).

16426-64-5
165 suppliers
$5.00/250mg

100959-22-6
144 suppliers
$14.00/1g
Yield:100959-22-6 99.5%
Reaction Conditions:
with thionyl chloride
Steps:
6 Methyl 2-bromo-4-hydroxybenzoate (10)
Example 6
Methyl 2-bromo-4-hydroxybenzoate (10)
2-Bromo-4-nitrobenzoic acid (6.2 g, 25.3 mmol) was dissolved in MeOH (120 mL), and SOCl2 (5.5 mL, 75.9 mmol) was added dropwise at 0° C.
The reaction mixture was refluxed for 6 h.
The reaction solvent was concentrated to dryness.
Residual SOCl2 was removed by co-evaporation with CH2Cl2 (*3).
The solid was dried under vacuum to afford methyl 2-bromo-4-nitrobenzoate in 99.5% (6.52 g) yield as a light yellow solid. 1H-NMR (CDCl3, 400 MHz) δ 8.50 (1H, s, Ar), 8.21 (1H, d, J=8.4 Hz, Ar), 7.91 (1H, d, J=8.4 Hz, Ar), 3.97 (3H, s, OCH3); 13C-NMR (CDCl3, 100 MHz) δ 165.2, 149.2, 137.9, 131.7, 129.1, 122.1, 122.0, 53.1; MS (ESI) m/z Calcd for C8H6BrNO4 (M+): 259.0, Found: 260.1 (M+H+).
References:
UNIVERSITY OF MARYLAND, BALTIMORE;FLETCHER, Steven US2014/256817, 2014, A1 Location in patent:Page/Page column

16426-64-5
165 suppliers
$5.00/250mg

74-88-4
357 suppliers
$15.00/10g

100959-22-6
144 suppliers
$14.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

16426-64-5
165 suppliers
$5.00/250mg

100959-22-6
144 suppliers
$14.00/1g
3558-19-8
32 suppliers
$90.00/250mg

100959-22-6
144 suppliers
$14.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
856794-72-4
1 suppliers
inquiry

100959-22-6
144 suppliers
$14.00/1g