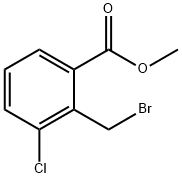
METHYL 2-BROMOMETHYL-3-CHLORO-BENZOATE synthesis
- Product Name:METHYL 2-BROMOMETHYL-3-CHLORO-BENZOATE
- CAS Number:188187-03-3
- Molecular formula:C9H8BrClO2
- Molecular Weight:263.52
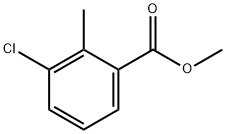
99586-84-2

188187-03-3
Methyl 3-chloro-2-methylbenzoate (1 g, 5.42 mmol) was used as a raw material, which was mixed with carbon tetrachloride (13.3 ml), N-bromosuccinimide (1.06 g, 5.96 mmol) and benzoyl peroxide (3.5 mg, 0.0108 mmol) to form a suspension. The reaction mixture was heated in an oil bath at 90°C under nitrogen protection. After the reaction was carried out for 3 hours and 45 minutes, the heating was stopped. The reaction solution was sequentially diluted with water, ethyl acetate and saturated aqueous sodium bicarbonate. The organic layer was separated, washed with saturated aqueous sodium bicarbonate solution, dried over anhydrous magnesium sulfate, filtered and concentrated to afford the target product methyl 2-bromomethyl-3-chloro-benzoate (1.43 g, yield: 100%). The product was characterized by 1H-NMR (400 MHz, CDCl3) with chemical shifts δppm of 4.00 (3H, s), 5.12 (2H, s), 7.32 (1H, t, J = 8 Hz), 7.58 (1H, dd, J = 2,8 Hz), 7.86 (1H, dd, J = 2,8 Hz), respectively.

99586-84-2
66 suppliers
$26.00/250mg

188187-03-3
82 suppliers
$60.00/250mg
Yield: 100%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane at 90; for 3.45 h;
Steps:
10c Methyl 2-(bromomethyl)-3-chlorobenzoate
A suspension of methyl 3-chloro-2-methylbenzoate obtained in Example 10b (1 g, 5.42 mmol), carbon tetrachloride (13.3 ml), N-bromosuccinimide (1.06 g, 5.96 mmol) and benzoyl peroxide (3.5 mg, 0.0108 mmol) was heated in a 90° C. oil bath under a nitrogen stream.
After three hours and 45 minutes, heating was completed, and the mixture was diluted with water, ethyl acetate and a saturated aqueous sodium bicarbonate solution.
The organic layer was washed with a saturated aqueous sodium bicarbonate solution, dried over anhydrous magnesium sulfate, filtered and concentrated to give the title compound (1.43 g, yield: 100%).
1H-NMR (400 MHz, CDCl3) δ ppm; 4.00 (3H, s), 5.12 (2H, s), 7.32 (1H, t, J=8 Hz), 7.58 (1H, dd, J=2, 8 Hz), 7.86 (1H, dd, J=2, 8 Hz).
References:
EISAI R&D MANAGEMENT CO., LTD.;YOSHIDA, Ichiro;OKABE, Tadashi;MATSUMOTO, Yasunobu;WATANABE, Nobuhisa;ONIZAWA, Yuji;OHASHI, Yoshiaki;HARADA, Hitoshi US2013/65925, 2013, A1 Location in patent:Paragraph 0338; 0339
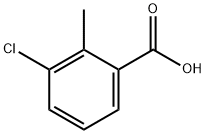
7499-08-3
190 suppliers
$8.00/10g

188187-03-3
82 suppliers
$60.00/250mg