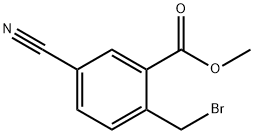
Methyl 2-(broMoMethyl)-5-cyanobenzoate synthesis
- Product Name:Methyl 2-(broMoMethyl)-5-cyanobenzoate
- CAS Number:421551-82-8
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
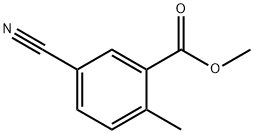
103261-68-3

421551-82-8
B) Synthesis of methyl 2-(bromomethyl)-5-cyanobenzoate Methyl 5-cyano-2-methylbenzoate (8.9 g), N-bromosuccinimide (9.5 g), 2,2'-azobis(isobutyronitrile) (0.83 g) were mixed with (trifluoromethyl)benzene (200 mL) and the reaction was stirred for 6 hours at 80 °C under nitrogen protection. Upon completion of the reaction, the mixture was poured into water, the organic layer was separated and dried over anhydrous sodium sulfate, and subsequently concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate) to afford the target product methyl 2-(bromomethyl)-5-cyanobenzoate (8.05 g). The product was characterized by 1H NMR (300 MHz, CDCl3) with chemical shifts of δ3.98 (3H, s), 4.96 (2H, s), 7.61 (1H, d, J = 7.9 Hz), 7.77 (1H, dd, J = 7.9,1.9 Hz), 8.27 (1H, d, J = 1.9 Hz).

103261-68-3
55 suppliers
inquiry

421551-82-8
58 suppliers
inquiry
Yield:421551-82-8 90%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane at 80; for 12 h;
Steps:
IV: To a solution of methyl 5-cyano-2-methyl-benzoate III (61 .0 g, 348 mmol, 1 .00 eq) and N-bromosuccinimide (75.8 g, 383 mmol, 1 .10 eq) in carbon tetrachloride (650 mL) was added benzoyl peroxide (8.43 g, 34.8 mmol, 0.100 eq). The mixture was stirred at 80 °Cfor 1 2 h. The mixture was concentrated in vacuum, suspended in water (500 mL) and then extracted with ethyl acetate (2 x 500 mL). The aqueous phase was extracted with ethyl acetate (2 x 300 mL). The combined organic phase was washed with brine (2 x 100 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by silica gel chromatography (petroleum ether/ethyl acetate = 0/1 to 5/1 ) to afford methyl 2-(bromomethyl)-5-cyano- benzoate IV (80.0 g, 31 5 mmol, 90% yield) as a yellow solid. 1H NMR (400 MHz, CDCl3-d) δ = 8.20 (d, J = 1 .6 Hz, 1 H), 7.70 (dd, J = 1 .8, 8.0 Hz, 1 H), 7.54 (d, J = 8.0 Hz, 1 H), 4.89 (s, 2H), 3.91 (s, 3H).
References:
WO2021/69705,2021,A1 Location in patent:Page/Page column 115-116
79669-50-4
199 suppliers
$12.00/5g

421551-82-8
58 suppliers
inquiry
42872-79-7
59 suppliers
$19.00/1g

421551-82-8
58 suppliers
inquiry
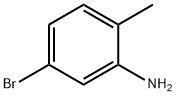
39478-78-9
324 suppliers
$10.00/5g

421551-82-8
58 suppliers
inquiry
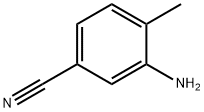
60710-80-7
137 suppliers
inquiry

421551-82-8
58 suppliers
inquiry