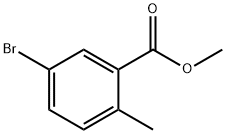
methyl 5-bromo-2-methyl-benzoate synthesis
- Product Name:methyl 5-bromo-2-methyl-benzoate
- CAS Number:79669-50-4
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

67-56-1
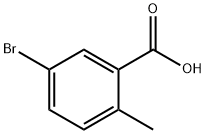
79669-49-1

79669-50-4
5-Bromo-2-methylbenzoic acid (1.0 g, 4.7 mmol) was placed in a 200 mL round-bottomed flask under nitrogen protection and methanol was added via syringe. A hexane solution of 2 M diazomethyltrimethylsilane (3.5 mL, 23.0 mmol) was slowly added dropwise over 10 min and the reaction mixture was stirred for 1 h at room temperature. Subsequently, glacial acetic acid (16 mL) was added and stirring was continued for 45 minutes. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (100 mL) and washed sequentially with 1 M aqueous sodium hydroxide solution (30 mL), saturated aqueous sodium bicarbonate solution (30 mL) and brine (30 mL). The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give methyl 5-bromo-2-methylbenzoate 1.01 g (99% yield). Methyl 5-bromo-2-methylbenzoate (1.04 g, 4.5 mmol) was placed in a 50 mL round-bottom flask under nitrogen protection and carbon tetrachloride (15 mL) was added. Subsequently, N-bromosuccinimide (1.49 g, 8.3 mmol) and 2,2'-azobisisobutyronitrile (40 mg, 0.2 mmol) were added, a condenser was mounted, and the reaction was refluxed for 4 hours. After completion of the reaction, it was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and the crude product was pre-adsorbed through silica gel. The residue was purified by silica gel column chromatography using a gradient elution with a hexane solution of dichloromethane (0 to 50%) to afford methyl 5-bromo-2-bromomethylbenzoate 977 mg (70% yield). Methyl 5-bromo-2-bromomethylbenzoate (0.984 g, 3.20 mmol) and the method described in Part I were used to replace the synthetic step of 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,3-dihydroisoindol-1-one, which was prepared to give 509 mg of methyl 2-methyl-5-bromobenzoate (75% yield).

67-56-1
790 suppliers
$7.29/5ml-f

79669-49-1
445 suppliers
$5.00/1g

79669-50-4
199 suppliers
$12.00/5g
Yield:79669-50-4 99%
Reaction Conditions:
Stage #1:methanol;5-bromo-2-methylbenzoic acid with diazomethyl-trimethyl-silane in hexane at 20; for 1.16667 h;
Stage #2: with acetic acid in hexane for 0.75 h;
Steps:
5
Place 5-bromo-2-methyl-benzoic acid (1.0 g, 4.7 mmol) in a 200 mL flask under an N2 atmosphere and add methanol via syringe. Add a 2M solution of diazomethyl- trimethyl-silane in hexane (3.5 mL, 23.0 mmol) drop wise over 10 minutes and stir for 1 hour at room temperature. Add glacial acetic acid (16 mL) and stir for 45 minutes. Dilute with ethyl acetate (100 mL) and wash with 1M aqueous sodium hydroxide solution (30 mL), saturated aqueous sodium bicarbonate solution (30 mL) and brine (30 mL). Dry the organic layer (Na2SO4), filter and concentrate in vacuo to obtain 1.01 g of 5-bromo-2- methyl-benzoic acid methyl ester (99 %). Place 5-bromo-2-methyl-benzoic acid methyl ester (1.04 g, 4.5 mmol) in a 50 mL flask under a N2 atmosphere and add carbon tetrachloride (15 mL). Add N-bromo- succinamide (1.49 g, 8.3 mmol) and 2,2'-azobisisobutyronitrile (40 mg, 0.2 mmol) and fit flask with a condenser and reflux for 4 hours. Cool to room temperature and filter. Concentrate the filtrate and pre-adsorb the crude product onto silica gel. Chromatograph the residue on a Si02 column eluting with dichloromethane in hexane (0 to 50%) to obtain 977 mg of 5-bromo-2-bromomethyl-benzoic acid methyl ester (70%). Using 5-bromo-2-bromomethyl-benzoic acid methyl ester (0. 984 g, 3.20 mmol) and the procedure described in the I st paragraph for the alternative procedure for 5- (4,4, 5, 5-tetramethyl- [1, 3,2] dioxaborolan-2-yl) -2, 3-dihydro-isoindol-1-one, prepare 509 mg of the title compound (75 %).
References:
ELI LILLY AND COMPANY WO2005/73205, 2005, A1 Location in patent:Page/Page column 27-28

79669-49-1
445 suppliers
$5.00/1g

74-88-4
356 suppliers
$15.00/10g

79669-50-4
199 suppliers
$12.00/5g

79669-49-1
445 suppliers
$5.00/1g

18107-18-1
210 suppliers
$33.00/5g

79669-50-4
199 suppliers
$12.00/5g

79669-49-1
445 suppliers
$5.00/1g

79669-50-4
199 suppliers
$12.00/5g
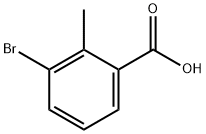
76006-33-2
321 suppliers
$10.00/5g

79669-49-1
445 suppliers
$5.00/1g

74-88-4
356 suppliers
$15.00/10g
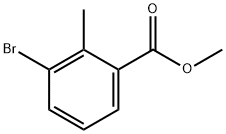
99548-54-6
171 suppliers
$5.00/1g

79669-50-4
199 suppliers
$12.00/5g