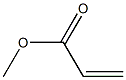
METHYL 2-NITROCINNAMATE synthesis
- Product Name:METHYL 2-NITROCINNAMATE
- CAS Number:39228-29-0
- Molecular formula:C10H9NO4
- Molecular Weight:207.18
Yield:39228-29-0 99%
Reaction Conditions:
with sulfuric acid at 70; for 12 h;
Steps:
General preparation of compound 2 (R1, R2 = H)
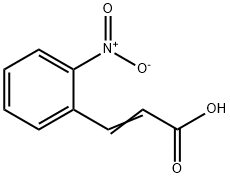
To a solution of 2-nitrocinnamic acid (1, 3.00 g, 15.53 mmol) in CH3OH (100 mL) was dropped conc. H2SO4 (0.25 mL) at rt, and the reaction mixture was stirred at reflux overnight and then allowed to cool to rt. A saturated solution of NaHCO3 was added and CH3OH was distilled off. The mixture was extracted with EtOAc, dried (MgSO4), filtered, and evaporated in vacuo to give the desired product 2 (3.21 g, >99%) as a colorless solid: mp 75 ; 1H NMR (400 MHz, CDCl3) d 8.10 (1H, d, J = 15.9 Hz), 8.03 (1H, d, J = 7.5 Hz, Ph), 7.70-7.63 (2H, m, Ph), 7.56 (1H, m, Ph), 6.37 (1H, d, J = 15.9 Hz), 3.83 (3H, s); 13C NMR (100 MHz, CDCl3) d 166.5, 148.6, 140.4, 133.9, 130.9, 130.7, 129.4, 125.2, 123.1, 52.3.
References:
Park, Byeongyeon;Nam, Ji Hye;Kim, Jin Han;Kim, Hyoung Ja;Onnis, Valentina;Balboni, Gianfranco;Lee, Kyung-Tae;Park, Jeong Ho;Catto, Marco;Carotti, Angelo;Lee, Jae Yeol [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 5,p. 1179 - 1185] Location in patent:supporting information