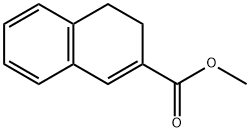
Methyl 3,4-Dihydronaphthalene-2-Carboxylate synthesis
- Product Name:Methyl 3,4-Dihydronaphthalene-2-Carboxylate
- CAS Number:51849-37-7
- Molecular formula:C12H12O2
- Molecular Weight:188.22
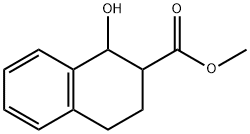
343949-61-1
0 suppliers
inquiry

51849-37-7
13 suppliers
$200.00/50mg
Yield:51849-37-7 93%
Reaction Conditions:
toluene-4-sulfonic acid in toluene; for 3 h;Heating / reflux;
Steps:
14.B
Step 14B: To a solution of alcohol 14a (2.63 g, 12.8 mmol) in toluene (30 mL) was added p-toluenesulfonic acid monohydrate (0.14 g, 0.71 mmol). The mixture was stirred under reflux conditions for 3 h then cooled to ambient temperature and diluted with dichloromethane (100 mL). The mixture was washed with de-ionized water (15 mL) and the organics were dried over magnesium sulfate, filtered and concentrated in vacuo to afford unsaturated ester 14b (2.25 g, 93%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.53 (s, 1H), 7.10-7.28 (m, 4H), 3.82 (s, 3H), 2.87 (m, 2H), 2.61 (m, 2H). APCI MS m/e: 189.0 ([M+H]+).
References:
US2006/276454,2006,A1 Location in patent:Page/Page column 32-33
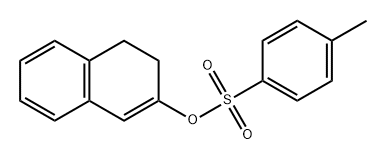
849943-93-7
0 suppliers
inquiry

67-56-1
776 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

51849-37-7
13 suppliers
$200.00/50mg
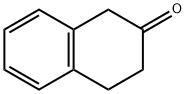
530-93-8
262 suppliers
$10.00/1g

51849-37-7
13 suppliers
$200.00/50mg

67946-45-6
0 suppliers
inquiry

51849-37-7
13 suppliers
$200.00/50mg

67-56-1
776 suppliers
$9.00/25ml
![1-Naphthalenol, 2-[bis(methylthio)methylene]-1,2,3,4-tetrahydro-](/CAS/20210305/GIF/87711-86-2.gif)
87711-86-2
0 suppliers
inquiry

51849-37-7
13 suppliers
$200.00/50mg