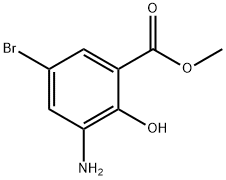
Methyl 3-amino-5-bromo-2-hydroxybenzoate 95+% synthesis
- Product Name:Methyl 3-amino-5-bromo-2-hydroxybenzoate 95+%
- CAS Number:141761-82-2
- Molecular formula:C8H8BrNO3
- Molecular Weight:246.06

67-56-1
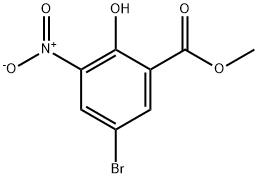
91983-31-2

141761-82-2
The general procedure for the synthesis of methyl 3-amino-5-bromo-2-hydroxybenzoate from methyl 5-bromo-2-hydroxy-3-nitrobenzoate (138.02 g, 0.5 mol) and methanol (1000 mL) was as follows: methyl 5-bromo-2-hydroxy-3-nitrobenzoate was mixed with methanol, followed by a one-time addition of activated iron powder (112 g, 2 mol). The reaction mixture was heated to a slightly refluxed state, saturated ammonium chloride solution (80 g, 1.5 mol) was slowly added dropwise and the reflux reaction was maintained for 3 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the reaction system was cooled to room temperature. Diatomaceous earth (200 g) was added to the cooled reaction mixture and filtration was carried out to obtain a black filtrate. The solid residue was washed well with hot methanol until no product was detected in the wash solution and the black solid was discarded. All the filtrates were combined and the solvent was removed by evaporation under reduced pressure. The crude product was purified by rapid chromatography on an 80-100 mesh silica gel column to afford methyl 3-amino-5-bromo-2-hydroxybenzoate (96-85 g) in 78% yield.

67-56-1
790 suppliers
$7.29/5ml-f

91983-31-2
34 suppliers
inquiry

141761-82-2
50 suppliers
$45.00/2.5mg
Yield: 78%
Reaction Conditions:
with iron;ammonium chloride for 3 h;Reflux;
Steps:
2.4 Step 4: Preparation of methyl 5-bromo-2-hydroxy-3-aminobenzoate
After mixing methyl 5-bromo-2-hydroxy-3-nitrobenzoate (138.02 g, 0.5 mol) with methanol (1000 mL),One-time addition of activated iron powder (112g, 2mol),After heating to a slightly refluxed state, saturated ammonium chloride solution (80 g, 1.5 mol) was slowly added dropwise and refluxed for 3 hours.After the completion of the reaction by TLC, the system was cooled to room temperature, mixed with diatomaceous earth (200 g), and suction filtered to obtain a black filtrate. After the solids were thoroughly washed with hot methanol to the product, the black solids were discarded. All filtratesCombine, evaporate the solvent under reduced pressure and purify by flash chromatography on an 80-100 mesh silica gel column.The objective compound 5-bromo-2-hydroxy-3-aminobenzoic acid methyl ester 96.0 g was obtained in a yield of 78%.
References:
Sichuan University;Puluo Pharmaceutical Co., Ltd.;Yu Luoting;Wei Yuquan CN107573336, 2018, A Location in patent:Paragraph 0099; 0100; 0101; 0102

91983-31-2
34 suppliers
inquiry

141761-82-2
50 suppliers
$45.00/2.5mg
4068-76-2
183 suppliers
$8.00/1g

141761-82-2
50 suppliers
$45.00/2.5mg
119-36-8
1051 suppliers
$5.00/10g

141761-82-2
50 suppliers
$45.00/2.5mg
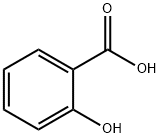
69-72-7
1316 suppliers
$5.00/10g

141761-82-2
50 suppliers
$45.00/2.5mg