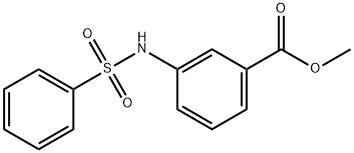
Methyl 3-benzenesulfonaMidobenzoate synthesis
- Product Name:Methyl 3-benzenesulfonaMidobenzoate
- CAS Number:107922-46-3
- Molecular formula:C14H13NO4S
- Molecular Weight:291.32
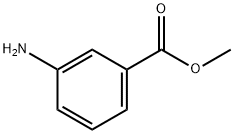
4518-10-9
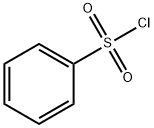
98-09-9

107922-46-3
Methyl 3-aminobenzoate (10 g, 66.2 mmol) was dissolved in pyridine (100 mL) and cooled in an ice bath for 15 minutes. Benzenesulfonyl chloride (8.53 mL, 66.2 mmol) was added slowly dropwise over 5 min using a syringe, followed by stirring the reaction mixture and gradually warming it to room temperature, the reaction lasted for 16 hours. The reaction mixture was slowly poured into a 1 M HCl solution (250 mL total volume) containing ice and the pH was adjusted to 2-3 to form a heterogeneous acidic mixture. The precipitate was collected by filtration and washed with plenty of water. The resulting solid was dried under high vacuum for 12 h to give the target product methyl 3-benzenesulfonylaminobenzoate (19.3 g, 100% yield) as an off-white solid.

4518-10-9
194 suppliers
$12.80/1g

98-09-9
364 suppliers
$12.00/25g

107922-46-3
30 suppliers
$27.00/100mg
Yield:107922-46-3 100%
Reaction Conditions:
with pyridine at 20; for 16 h;Inert atmosphere;Cooling with ice;
Steps:
7.e.1
Methyl 3-aminobenzoate (10 g, 66.2 mmol) was dissolved in pyridine (100 mL) and cooled in an ice bath for 15 min. Benzenesulfonyl chloride (8.53 ml, 66.2 mmol) was added via syringe over 5 min, and the solution then stirred with warming to room temperature over a 16 h period. The reaction mixture was poured onto ice and IM HCl (250 mL total volume) to afford a heterogenous acidic mixture (pH 2-3). The solid was filtered and washed with water. The collected solid was dried under hi-vacuum for 12 h to afford the coupled product (19.3, 100%) as an off-white solid.
References:
WO2010/126922,2010,A1 Location in patent:Page/Page column 26
618-95-1
213 suppliers
$14.00/5g

98-80-6
743 suppliers
$5.00/5g

107922-46-3
30 suppliers
$27.00/100mg
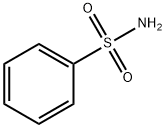
98-10-2
358 suppliers
$18.00/25g
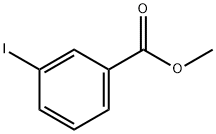
618-91-7
138 suppliers
$11.00/5g

107922-46-3
30 suppliers
$27.00/100mg
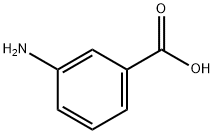
99-05-8
543 suppliers
$14.00/5g

107922-46-3
30 suppliers
$27.00/100mg