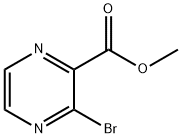
methyl 3-bromopyrazine-2-carboxylate synthesis
- Product Name:methyl 3-bromopyrazine-2-carboxylate
- CAS Number:51171-02-9
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
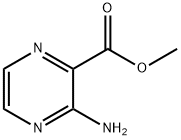
16298-03-6

51171-02-9
General procedure for the synthesis of methyl methyl 3-bromopyrazine-2-carboxylate from methyl 3-aminopyrazine-2-carboxylate: bromine (3.91 g, 24.46 mmol) was added slowly and dropwise at 0 °C to a stirred mixture containing methyl 3-aminopyrazine-2-carboxylate (1.27 g, 8.29 mmol) and hydrobromic acid (4.70 mL, 41.5 mmol). . Subsequently, a solution of sodium nitrite (1.44 g, 20.9 mmol) dissolved in water (6 mL) was added dropwise. The reaction mixture was continued to be stirred at 0 °C for 15 min. Upon completion of the reaction, the pH was adjusted to 8 with saturated aqueous sodium bicarbonate solution, followed by extraction with ethyl acetate (80 mL) and chloroform (50 mL). The organic phases were combined, dried with anhydrous magnesium sulfate, and concentrated under reduced pressure to afford 1.13 g (63% yield) of an orange oil, which solidified to the target product, methyl methyl-3-bromopyrazine-2-carboxylate, upon standing.

16298-03-6
335 suppliers
$7.00/1g

51171-02-9
113 suppliers
$21.00/250mg
Yield: 63%
Reaction Conditions:
with hydrogen bromide;bromine;sodium nitrite in water at 0; for 0.25 h;
Steps:
H.2 Step 2. Preparation of 3-bromopyrazine-2-carboxylic acid methyl ester
Bromine (3.91 g, 24.46 mmol) was added dropwise to a stirred mixture of 3- aminopyrazine-2-carboxylic acid methyl ester (1.27 g, 8.29 mmol) and hydrobromic acid (4.70 mL, 41.5 mmol) at 00C. A solution of sodium nitrite (1.44 g, 20.9 mmol) in water (6 mL) was then added dropwise. The reaction mixture was stirred for 15 min, brought to pH 8 with NaHCO3 (saturated, aqueous), and extracted with ethyl acetate (80 mL) and chloroform (50 mL). The combined organics were dried over MgSO4 and concentrated in vacuoto give 1.13 g (63%) of an orange oil which solidified on standing.
References:
BAYER PHARMACEUTICALS CORPORATION WO2006/91506, 2006, A2 Location in patent:Page/Page column 47
5424-01-1
345 suppliers
$10.00/5g

51171-02-9
113 suppliers
$21.00/250mg