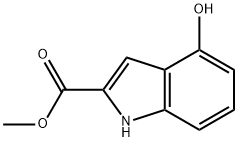
Methyl 4-hydroxy-1H-indole-2-carboxylate synthesis
- Product Name:Methyl 4-hydroxy-1H-indole-2-carboxylate
- CAS Number:27748-08-9
- Molecular formula:C10H9NO3
- Molecular Weight:191.18
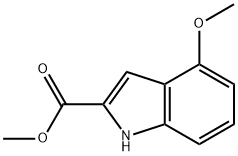
111258-23-2
150 suppliers
$21.00/100mg

27748-08-9
25 suppliers
$45.00/10mg
Yield:27748-08-9 88%
Reaction Conditions:
Stage #1: methyl 4-methoxy-1H-indole-2-carboxylatewith boron tribromide in dichloromethane at 0; for 2 h;
Stage #2: with water;sodium hydrogencarbonate in dichloromethane; pH=7;
Steps:
1.A
To an ice cold solution of 4-Methyloxy-1 H-indole-2-carboxylic acid methyl ester (1 g, 4.87 mmol) in DCM (10 ml) is added BBr3 (1M in DCM, 4.9 ml, 4.9 mmol). It is stirred for 1 hour and another equivalent (4.9 ml) of BBr3 is added. After another hour, the mixture is poured on ice and the pH is adjusted to 7 with sodium bicarbonate. Extraction with DCM gave a yellow powder. Yield : 0. 82 g (88%). MS (ESI) : 192.0 [M+H] +, 1 H-NMR (DMSO-d6) : No. (ppm) 11.78 (br s, 1 H), 9. 73 (s, 1H), 7.21 (d, 1H), 7.05 (dd, 1H), 6.89 (d, 1H), 6.4 (d, 1H), 3.86 (s, 3H).
References:
WO2005/77932,2005,A2 Location in patent:Page/Page column 60
27748-09-0
26 suppliers
$200.00/25mg

27748-08-9
25 suppliers
$45.00/10mg
![2-Propenoic acid, 2-azido-3-[2-(phenylmethoxy)phenyl]-, methyl ester](/CAS/20211123/GIF/117175-27-6.gif)
117175-27-6
0 suppliers
inquiry

27748-08-9
25 suppliers
$45.00/10mg
90-02-8
477 suppliers
$9.00/1g

27748-08-9
25 suppliers
$45.00/10mg