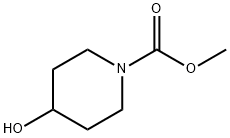
Methyl 4-hydroxypiperidine-1-carboxyl synthesis
- Product Name:Methyl 4-hydroxypiperidine-1-carboxyl
- CAS Number:75250-52-1
- Molecular formula:C7H13NO3
- Molecular Weight:159.18
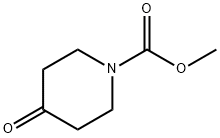
29976-54-3
34 suppliers
$29.00/100mg

75250-52-1
20 suppliers
$126.00/500mg
Yield: 44%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0; for 2 h;
Steps:
b (b) 4-Hydroxy-piperidine-1-carboxylic acid methyl ester 1-117
To a solution of 4-oxo-piperidine-1 -carboxylic acid methyl ester 1-115 (315 mg, 2.0 mmol) in MeOH (5 mL) at 0°C was added sodium borohydride (1 14 mg, 3.0 mmol). The reaction mixture was stirred at 0°C for 2 h. The reaction was quenched with saturated aqueous ammonium chloride (5 mL), MeOH was removed in vacuo and the aqueous layer was extracted with DCM (x 3). The combined organic extracts were passed through a phase separator cartridge (Biotage) and concentrated in vacuo to afford the crude product which was purified by column chromatography (Biotage, SP1 , 25 g KP-Sil, eluting with isohexane to EtOAc) to afford title compound as a colourless oil (140 mg, 0.88 mmol, 44%).
References:
AUSPHERIX LIMITED;HOLMES, Ian;NAYLOR, Alan;ALBER, Dagmar;POWELL, Jonathan Raymond;MAJOR, Meriel Ruth;NEGOITA-GIRAS, Gabriel;ALLEN, Daniel Rees;GUETZOYAN, Lucie Juliette;KING, Nigel Paul WO2017/93543, 2017, A2 Location in patent:Page/Page column 117
5382-16-1
0 suppliers
$9.00/5 g
79-22-1
0 suppliers
$38.00/1000g

75250-52-1
20 suppliers
$126.00/500mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

75250-52-1
20 suppliers
$126.00/500mg

75250-59-8
1 suppliers
inquiry

75250-52-1
20 suppliers
$126.00/500mg
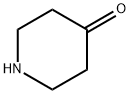
41661-47-6
0 suppliers
$45.00/250 mg

75250-52-1
20 suppliers
$126.00/500mg