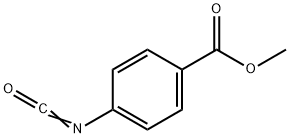
METHYL 4-ISOCYANATOBENZOATE 98 synthesis
- Product Name:METHYL 4-ISOCYANATOBENZOATE 98
- CAS Number:23138-53-6
- Molecular formula:C9H7NO3
- Molecular Weight:177.16
Yield: 96%
Reaction Conditions:
in 1,4-dioxane for 8 h;Reflux;
Steps:
5.2.1. Compound 1a
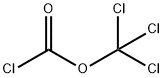
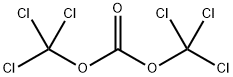
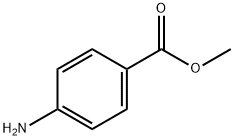
To methyl 4-aminobenzoate (30.23 g; 200 mmol) in anhydrous 1,4-dioxane (500 mL) was added trichloromethyl chloroformate (59.35 g; 300 mmol) dropwise and the reaction mixture was refluxed for 8 h. The solvent was removed on the rotary evaporator to yield 1a as a yellow solid, which was purified by vacuum distillation to give a yellow solid (34 g, 96% yield). IR (neat) νNCO 2268 cm-1. 1H NMR (CDCl3): δ 3.88 (s, 3H), 7.14-7.18 (m, 2H), 7.96-8.02 (m, 2H).
References:
Aravapalli, Sridhar;Lai, Huiguo;Teramoto, Tadahisa;Alliston, Kevin R.;Lushington, Gerald H.;Ferguson, Eron L.;Padmanabhan;Groutas, William C. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 13,p. 4140 - 4148] Location in patent:experimental part
32315-10-9
441 suppliers
$10.00/1g
619-45-4
386 suppliers
$6.00/1g

23138-53-6
69 suppliers
$149.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

23138-53-6
69 suppliers
$149.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

288-32-4
1040 suppliers
$5.00/25g

23138-53-6
69 suppliers
$149.00/5g

619-45-4
386 suppliers
$6.00/1g

23138-53-6
69 suppliers
$149.00/5g