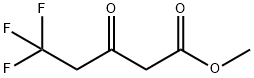
METHYL 5,5,5-TRIFLUORO-3-OXOPENTANOATE synthesis
- Product Name:METHYL 5,5,5-TRIFLUORO-3-OXOPENTANOATE
- CAS Number:915213-24-0
- Molecular formula:C6H7F3O3
- Molecular Weight:184.11
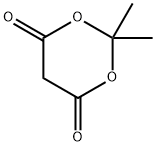
2033-24-1
660 suppliers
$6.00/25g
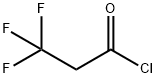
41463-83-6
71 suppliers
$25.00/1g

915213-24-0
27 suppliers
inquiry
Yield:915213-24-0 120 mg
Reaction Conditions:
with pyridine;dmap in dichloromethane at 0 - 20; for 3 h;Inert atmosphere;
Steps:
34.A Step A: 5,5,5-Trifluoro-3-oxo-pentanoic acid methyl ester
To a stirred solution of 2,2-dimethyl-[l,3]dioxane-4,6-dione (200 mg, 1.41 mmol), pyridine (0.125 ml, 1.55 mmol) and DMAP (344 mg, 2.81 mmol) in dichloromethane (10 ml), was added 3,3,3-trifluoro-propionyl chloride (0.16 ml, 1.55 mmol) at 0°C. The resulting reaction mixture was stirred under nitrogen for 1 h at 0°C and then allowed to warm to room temperature and stirred for 2 h. Volatiles were removed under reduced pressure, and the crude residue was diluted with diethyl ether and washed with 1 N HC1. The organic layer was dried and concentrated to get the intermediate, which was diluted with 10 ml of methanol and refluxed for 5 h. Volatiles were removed under reduced pressure to afford the crude 5,5,5-trifluoro-3-oxo-pentanoic acid methyl ester (120 mg, 46%) as brown liquid which was used in the following step without further purification. FIA-MS: 183 (M-H)~.
References:
WO2013/64465,2013,A1 Location in patent:Page/Page column 84-85

67-56-1
790 suppliers
$9.00/25ml

2033-24-1
660 suppliers
$6.00/25g

41463-83-6
71 suppliers
$25.00/1g

915213-24-0
27 suppliers
inquiry