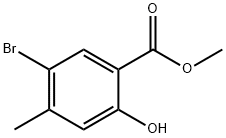
METHYL 5-BROMO-2-HYDROXY-4-METHYLBENZOATE synthesis
- Product Name:METHYL 5-BROMO-2-HYDROXY-4-METHYLBENZOATE
- CAS Number:39503-57-6
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07

67-56-1
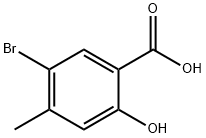
6623-35-4

39503-57-6
B) General procedure for synthesizing methyl 5-bromo-2-hydroxy-4-methylbenzoate: sulfuric acid (6.77 mL) was added slowly and dropwise to a solution of methanol (200 mL) containing 5-bromo-2-hydroxy-4-methylbenzoic acid (6.78 g). The reaction mixture was stirred continuously at 70 °C overnight. Upon completion of the reaction, the reaction mixture was concentrated by distillation under reduced pressure. Subsequently, the residue was neutralized with saturated aqueous sodium bicarbonate solution and extracted with ethyl acetate under ice bath cooling conditions. The organic layers were combined, washed sequentially with water and saturated brine, and then dried over anhydrous sodium sulfate. After drying, the solvent was removed by evaporation under reduced pressure. Finally, the residue was purified using silica gel column chromatography (eluent: ethyl acetate/hexane) to afford the target product methyl 5-bromo-2-hydroxy-4-methylbenzoate (6.56 g). The result of mass spectrometry analysis: [M-H]+ 243.0.
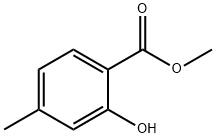
4670-56-8
177 suppliers
$10.00/1g

39503-57-6
65 suppliers
$8.00/250mg
Yield: 98.1%
Reaction Conditions:
with bromine in chloroform at 20; for 3 h;
Steps:
Intermediate 14: Methyl 5-bromo-2-hydroxy-4-methylbenzoate (1-14)
To a solution of methyl 2-hydroxy-4-methyl-benzoate (10.6 g, 0.06 mol) in chloroform (20 mL) was added dropwise bromine (3.27 mL, 0.06 mol) at 0°C and the mixture was stirred at room temperature for 3 h. The reaction mixture was concentrated under reduced pressure, the residue was neutralized with sodium sulfite solution under ice-cooling, pH 8,and the mixture was extracted with dichloromethane (100 mL x 3). Organic layer was washed with brine solution (50 mL) and dried over Na2SO4 and the solvent was concentrated under vacuum to obtain the crude compound which was further purified by flash chromatography using n-hexane: ethyl acetate (97:3) to afford the title compound. Yield: 15 g (98.1%); ‘H -NMR (CDC13, 400 MHz) ö ppm: 2.38 (s, 3H), 3.94 (s, 3H), 6.88 (s, 1H), 7.96 (s, 1H), 10.58(s, 1H); Mass (mlz): 244.8 (M+H).
References:
SUVEN LIFE SCIENCES LIMITED;NIROGI, Ramakrishna;MOHAMMED, Abdul Rasheed;SHINDE, Anil Karbhari;GAGGINAPALLY, Shankar Reddy;KANCHARLA, Durga Malleshwari;PANDEY, Santosh Kumar;ABRAHAM, Renny;JASTI, Venkateswarlu WO2019/82140, 2019, A1 Location in patent:Page/Page column 22; 23

67-56-1
790 suppliers
$7.29/5ml-f

6623-35-4
41 suppliers
$18.00/100mg

39503-57-6
65 suppliers
$8.00/250mg

6623-35-4
41 suppliers
$18.00/100mg

39503-57-6
65 suppliers
$8.00/250mg

6623-35-4
41 suppliers
$18.00/100mg

74-88-4
357 suppliers
$15.00/10g

39503-57-6
65 suppliers
$8.00/250mg
50-85-1
345 suppliers
$10.00/5g

39503-57-6
65 suppliers
$8.00/250mg