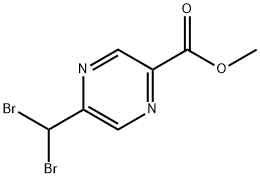
Methyl 5-(dibromomethyl)pyrazine-2-carboxylate synthesis
- Product Name:Methyl 5-(dibromomethyl)pyrazine-2-carboxylate
- CAS Number:866327-72-2
- Molecular formula:C7H6Br2N2O2
- Molecular Weight:309.94
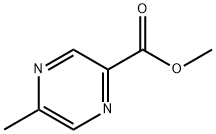
41110-33-2

866327-72-2
Methyl 5-methylpyrazine-2-carboxylate (1.60 g, 10.5 mmol) was used as a raw material and was mixed with N-bromosuccinimide (5.62 g) and dibenzoyl peroxide (0.255 g, 1.05 mmol) according to the method of Macdonald, SJF et al. (J. Med. Chem. 2002, 45(18):3878-3890) in carbon tetrachloride (80 mL). ) were mixed in carbon tetrachloride (80 mL). The reaction mixture was heated to reflux for 18 hours. After completion of the reaction, the mixture was cooled and washed sequentially with 10% aqueous sodium sulfite (2 x 20 mL) and water (1 x 30 mL). The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated to give a brown oily crude product (2.41 g). The crude product was purified by silica gel column chromatography (eluent: 0-20% ethyl acetate/hexane) to afford the target compound methyl 5-(dibromomethyl)pyrazine-2-carboxylate (1.00 g, 31% yield).

41110-33-2
125 suppliers
$6.00/250mg

866327-72-2
14 suppliers
inquiry
Yield:866327-72-2 31%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane; for 18 h;Heating / reflux;
Steps:
23.A
5-Methyl-pyrazine-2-carboxylic acid methyl ester (1.60 g, 10.5 mmol; Macdonald, S. J. F. et al. J.Med.Chem. 2002, 45(18):3878-3890.), N-bromosuccinimide (5.62 g, 31.6 mmol), and dibenzoyl peroxide (0.255 g, 1.05 mmol) were dissolved in CCl4 (80 mL). The mixture was heated at reflux for 18 h. The reaction mixture was cooled and washed with 10% aq. Na2SO3 (2×20 mL) and H2O (1×30 mL). The organic phase was dried over Na2SO4, filtered, and concentrated to yield a brown oily crude material (2.41 g). The crude product was purified (SiO2: 0-20% ethyl acetate/hexanes) to give the title compound (1.00 g, 31%).
References:
US2005/222151,2005,A1 Location in patent:Page/Page column 19

41110-33-2
125 suppliers
$6.00/250mg
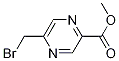
193966-70-0
50 suppliers
$45.00/10mg

866327-72-2
14 suppliers
inquiry
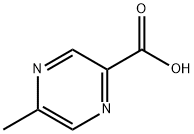
5521-55-1
461 suppliers
$9.00/5g

866327-72-2
14 suppliers
inquiry